Targeted Synthesis and Antitumor Activity In Vitro Macrodiolides Containing 1Z,5Z-Diene and 1,3-Diyne Moieties †
Abstract
:1. Introduction
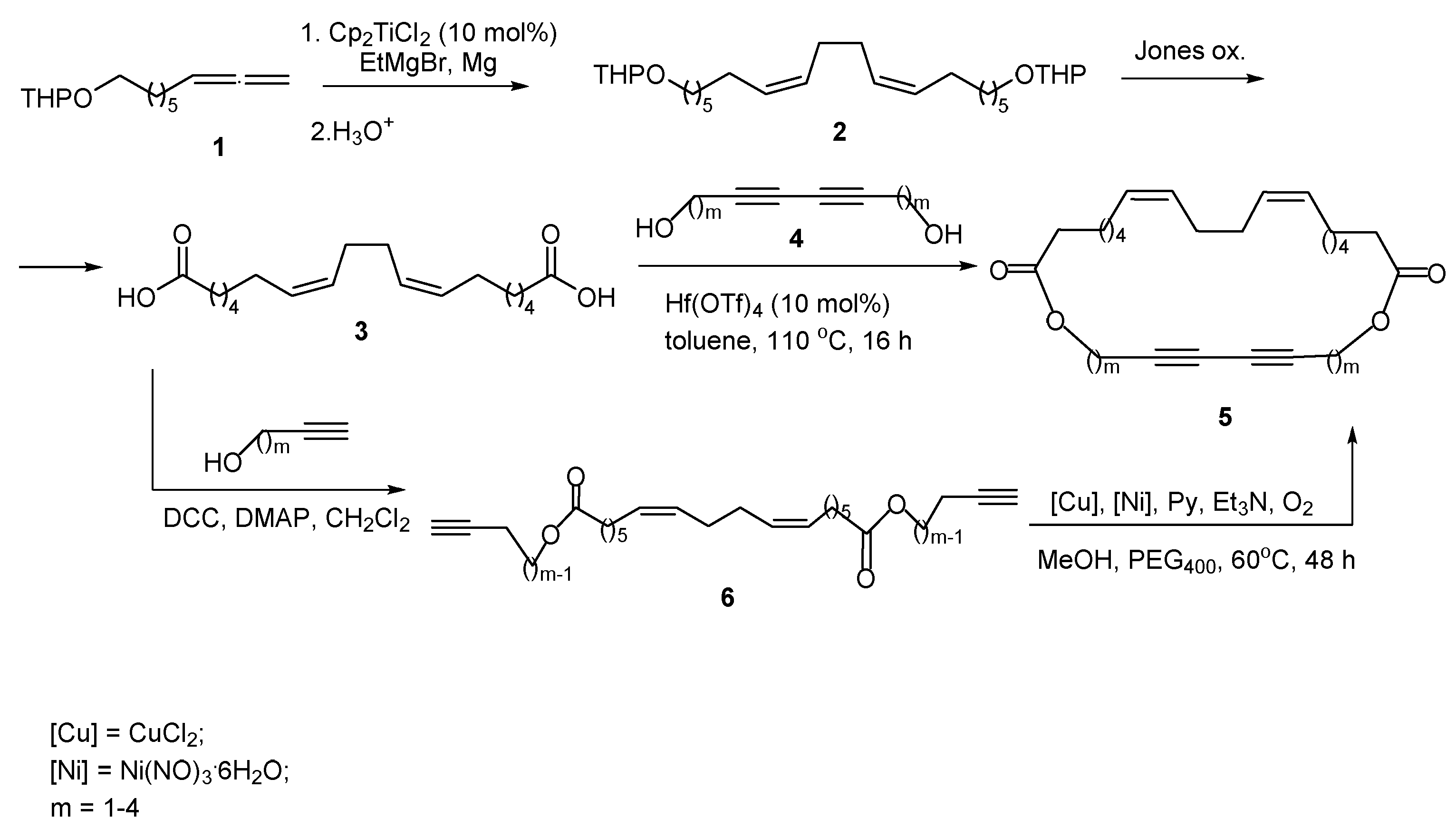
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.K. Macrocycles: Lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015, 6, 30–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.K.; Meher, R.K.; Reddy, P.K.; Pedapati, R.K.; Pragyandipta, P.; Kantevari, S.; Naik, M.R.; Naik, P.K. Rational design, chemical synthesis and cellular evaluation of novel 1,3-diynyl derivatives of noscapine as potent tubulin binding anticancer agents. J. Mol. Graph. Model. 2021, 106, 107933. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Wright, M.H.; Sieber, S.A. Making a long journey short: Alkyne functionalization of natural product scaffolds. Chem. Eur. J. 2016, 22, 4666–4678. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lei, A. 1,3-Diyne chemistry: Synthesis and derivations. Tetrahedron Lett. 2014, 55, 2763–2772. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.Q.; Miao, Y.H.; Li, X.; Zhou, Y.Z.; Gao, X.X.; Zhang, X.; Qin, X.M. Discovery of 1,3-diyne compounds as novel and potent antidepressant agents: Synthesis, cell-based assay and behavioral studies. RSC Adv. 2017, 7, 16005–16014. [Google Scholar] [CrossRef] [Green Version]
- Erwin, A.L. Antibacterial drug discovery targeting the lipopolysaccharide biosynthetic enzyme LpxC. Cold Spring Harb. Perspect. Med. 2016, 6, a025304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubaidullin, R.R.; Khalitova, R.R.; Nedopekina, D.A.; Spivak, A.Y. Homo-and cross coupling of C-2 propargyl substituted triterpenoic acids: Synthesis of novel symmetrical and unsymmetrical triterpene 1,3-diynes. Chem. Sel. 2018, 3, 13526–13529. [Google Scholar] [CrossRef]
- Sari, O.; Roy, V.; Balzarini, J.; Snoeck, R.; Andrei, G.; Agrofoglio, L.A. Synthesis and antiviral evaluation of C5-substituted-(1,3-diyne)-2′ -deoxyuridines. Eur. J. Med. Chem. 2012, 53, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Yang, S.P.; Zhou, Y.; Wu, W.B.; Tang, W.; Zuo, J.-P.; Li, Y.; Yue, J.M. Ivorenolide A, an Unprecedented Immunosuppressive Macrolide from Khaya ivorensis: Structural Elucidation and Bioinspired Total Synthesis. J. Am.Chem. Soc. 2012, 134, 20605–20608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.-F.; Xue, J.-J.; Zhou, Y.; Yu, H.-C.; Yang, S.-P.; Zhang, B.; Zuo, J.-P.; Li, Y.; Yue, J.-M. Ivorenolide B, an Immunosuppressive 17-Membered Macrolide from Khaya ivorensis: Structural Determination and Total Synthesis. Org. Lett. 2014, 16, 2062–2065. [Google Scholar] [CrossRef] [PubMed]
- Schaubach, S.; Gebauer, K.; Ungeheuer, F.; Hoffmeister, L.; Ilg, M.K.; Wirtz, C.; Furstner, A. A Two-Component Alkyne Metathesis Catalyst System with an Improved Substrate Scope and Functional Group Tolerance: Development and Applications to Natural Product Synthesis. Chem. Eur. J. 2016, 22, 8494–8507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, D.K.; Umamaheshwar, G.; Rao, R.N.; Rao, T.S.; Kumar, S.; Yadav, J.S. Total Synthesis of Ivorenolide A Following a Base-Induced Elimination Protocol. Org. Lett. 2015, 17, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Agbedahunsi, J.M.; Fakoya, F.A.; Adesanya, S.A. Studies on the anti-inflammatory and toxic effects of the stem bark of Khaya ivorensis (Meliaceae) on rats. Phytomedicine 2004, 11, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Czako, B.; Kurti, L. Molecules and Medicine; Wiley: New York, NY, USA, 2008; 272p. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Khusainova, E.M.; Dzhemilev, U.M. Original catalytic synthesis of macrodiolides containing a 1Z,5Z-diene moiety. Mendeleev Commun. 2018, 28, 503–504. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Khusainova, E.M.; Yunusbaeva, M.M.; Dzhemilev, U.M. Targeted synthesis of macrodiolides containing bis-methylene-separated Z-double bonds and their antitumor activity in vitro. Tetrahedron 2018, 74, 4606–4612. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorg. Chem. 2020, 99, 103832. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Yunusbaeva, M.M.; Dzhemilev, U.M. Stereoselective synthesis and antitumor activity of macrodiolides containing 1Z,5Z-diene and 1,3-diyne moieties. Mendeleev Commun. 2019, 29, 613–615. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, I.; Yusupova, A.; Dzhemileva, L.U.; Dzhemilev, U. Targeted Synthesis and Antitumor Activity In Vitro Macrodiolides Containing 1Z,5Z-Diene and 1,3-Diyne Moieties. Chem. Proc. 2022, 8, 6. https://doi.org/10.3390/ecsoc-25-11704
Islamov I, Yusupova A, Dzhemileva LU, Dzhemilev U. Targeted Synthesis and Antitumor Activity In Vitro Macrodiolides Containing 1Z,5Z-Diene and 1,3-Diyne Moieties. Chemistry Proceedings. 2022; 8(1):6. https://doi.org/10.3390/ecsoc-25-11704
Chicago/Turabian StyleIslamov, Ilgiz, Adelya Yusupova, Lilya U. Dzhemileva, and Usein Dzhemilev. 2022. "Targeted Synthesis and Antitumor Activity In Vitro Macrodiolides Containing 1Z,5Z-Diene and 1,3-Diyne Moieties" Chemistry Proceedings 8, no. 1: 6. https://doi.org/10.3390/ecsoc-25-11704
APA StyleIslamov, I., Yusupova, A., Dzhemileva, L. U., & Dzhemilev, U. (2022). Targeted Synthesis and Antitumor Activity In Vitro Macrodiolides Containing 1Z,5Z-Diene and 1,3-Diyne Moieties. Chemistry Proceedings, 8(1), 6. https://doi.org/10.3390/ecsoc-25-11704






