Synthesis and Antibacterial Activity of Thymyl Ethers †
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Section
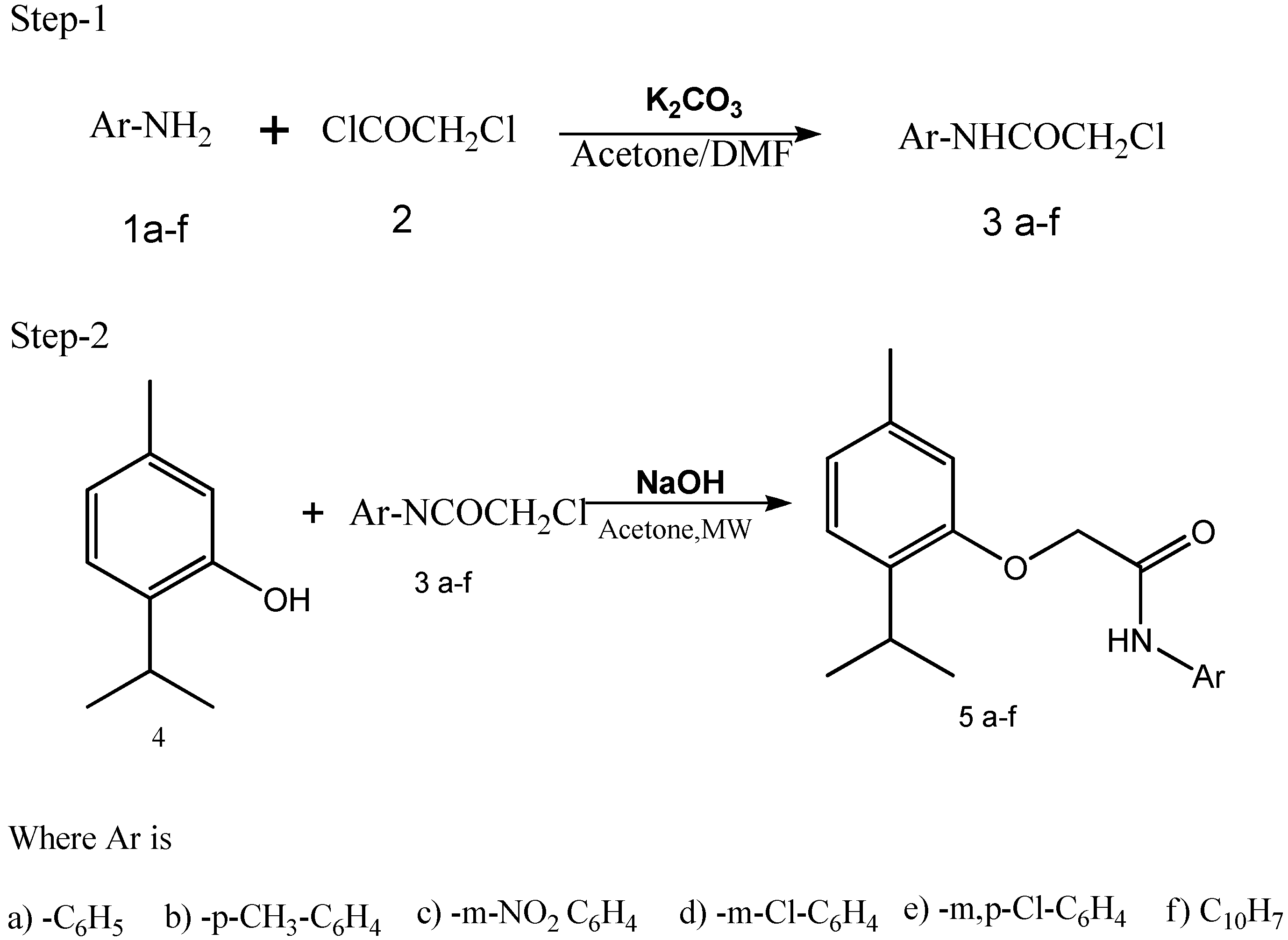
3.1. Synthesis of N-chloro Acetyl Aryl Amines (𝛼-Chloro Acetanilides)
3.2. Synthesis of Thymyl Ethers Using Microwave Method
3.3. General Procedure
3.4. Compounds and Their Spectral Data
3.5. Antibacterial Activity
4. Results and Discussion
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewang, P.M.; Nikumbh, V.P.; Tare, V.S.; Mahulikar, P.P. Eco-friendly pest management using monoterpenoirls II—antifungal efficacy of menthol derivative. J. Sci. Ind. Res. 2003, 62, 990. [Google Scholar]
- Kumbhar, P.P.; Dewang, P.M. Eco-friendly Pest Management Using Monoterpenoids. I. Antifungal Efficacy of Thymol Derivatives. J. Sci. Ind. Res. 2001, 60, 645. [Google Scholar]
- Coats, J.R.; Karr, L.L.; Drewes, C.D. Naturally Occuring Pest Bioregulators. In ACS Symposium Series 449; Paul, A.H., Ed.; American Chemical Society: Washington, DC, USA, 1991; p. 305. [Google Scholar]
- Tsao, R.; Lee, S.; Rice, P.J.; Jensen, C.; Coats, J.R. Synthesis and Chemistry of Agrochemicals IV. In ACS Symposium Series 584; Baker, D.R., Ed.; American Chemical Society: Washington, DC, USA, 1995; pp. 312–324. [Google Scholar]
- Duke, S.O. Handbook of Natural Toxins; Marcel Dekker Inc.: New York, NY, USA, 1991; Volume 6, Chapter 13; p. 269. [Google Scholar]
- Dev, S.; Narula, A.P.S.; Yadav, J.S. CRC Handbook of Terpenoids; CRC Press Inc.: Boca Raton, FL, USA, 1982; Volume 1, p. 7. [Google Scholar]
- Whittaker, R.H. Chemical Ecology; Academic Press: New York, NY, USA, 1970; p. 43. [Google Scholar]
- Rice, P.J.; Coats, J.R. Bioregulators for Crop Protection and Pest Control. In ACS Symposium Series 557; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1994; p. 92. [Google Scholar]
- More, D.H.; Pawar, N.S.; Dewang, P.M.; Patil, S.L.; Mahulikar, P.P. Microwave-assisted Sinthesis of Thymyl Ethers and Esters in Aqueous Medium. Rus. J. Gen. Chem. 2004, 74, 217. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Nema, A.; Srivastava, S.D. Conventional as well as microwave assisted synthesis of some new N⁹-[hydrazinoacetyl-(2-oxo-3-chloro-4-substituted aryl azetidine)]-carbazoles: Antifungal and antibacterial studies. Ind. J. Chem. 2008, 47, 606. [Google Scholar] [CrossRef]
- Patel, R.B.; Chikhalia, K.H. Synthesis of heterocyclic and non-heterocyclic entities as antibacterial and anti-HIV agents. Ind. J. Chem. 2006, 45, 1871. [Google Scholar] [CrossRef]
- Pattan, S.R.; Ali, M.S.; Pattan, J.S.; Purohit, S.S.; Reddy, V.V.K.; Natraj, B.R. Synthesis and microbiological evaluation of 2-acetanilido-4-arylthiazole derivatives. Ind. J. Chem. 2006, 45, 1929. [Google Scholar] [CrossRef]
- Varma, R.S. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002; p. 181. [Google Scholar]
- Varma, R.S. Solvent-free accelerated organic syntheses using microwaves. Pure Appl. Chem. 2001, 73, 193. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43. [Google Scholar] [CrossRef]
- Varma, R.S. Clay and clay-supported reagents in organic synthesis. Tetrahedral 2002, 58, 1235. [Google Scholar] [CrossRef]
- Wei, W.; Keh, C.C.K.; Li, C.J.; Varma, R.S. Water as a reaction medium for clean chemical processes. Clean Tech. Environ. Policy 2004, 7, 62. [Google Scholar] [CrossRef] [Green Version]
| Compounds | Ar | Molecular Formula | M.P. (°C) | Reaction Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 3a | -C6H5 | C8H8NOCl | 87–91 | 2.0 | 88 |
| 3b | -p-CH3C6H4 | C9H10NOCl | 163–93 | 3.0 | 91 |
| 3c | -m-NO2C6H4 | C8H7N2O3Cl | 90–93 | 2.0 | 82 |
| 3d | -m-ClC6H4 | C8H7NOCl2 | 87–92 | 2.5 | 86 |
| 3e | -m,p-ClC6H3 | C8H6NOCl3 | 97–101 | 2.0 | 84 |
| 3f | -C10H7 | C12H10NOCl | 155–157 | 3.0 | 80 |
| Compounds a | Ar | Molecular Formula | M.P. (°C) | Reaction Time | Yields b | ||
|---|---|---|---|---|---|---|---|
| Conventional (h) | M W (min) | Conventional (%) | MW (%) | ||||
| 5a | -C6H5 | C18H21NO2 | 80 | 4.0 | 1.5 | 50 | 91 |
| 5b | -p-CH3 C6H4 | C19H23NO2 | 77 | 4.5 | 1.0 | 47 | 90 |
| 5c | -m-NO2 C6H4 | C18H20N2O4 | 107 | 5.0 | 2.0 | 48 | 94 |
| 5d | -m-Cl C6H4 | C18H20NO2Cl | 72 | 4.5 | 2.5 | 57 | 91 |
| 5e | -m,p-Cl2 C6H3 | C18H19NO2Cl2 | 65 | 5.0 | 1.5 | 60 | 89 |
| 5f | -C10H7 | C22H23NO2 | 115 | 4.5 | 1.5 | 61 | 91 |
| Compounds | Zone of Inhibition in mm at Concentration of 20 mg/mL | |||
|---|---|---|---|---|
| P. vulgaries | S. aureus | E. coli | B. subtilis | |
| Aniline | ---- | 25 | ---- | 25 |
| 3a | 07 | 25 | 10 | 18 |
| 3b | 09 | 28 | ---- | 12 |
| 3c | 14 | ---- | 07 | 13 |
| 3d | 18 | 20 | ---- | 25 |
| 3e | 14 | 34 | ---- | 20 |
| 3f | ---- | 23 | ---- | 20 |
| Thymol | ---- | ---- | ---- | 10 |
| 5a | 06 | ---- | ---- | ---- |
| 5b | 09 | ---- | 05 | ---- |
| 5c | 14 | 31 | ---- | ---- |
| 5d | 06 | ---- | ---- | 10 |
| 5e | ---- | ---- | ---- | 06 |
| 5f | 05 | 05 | ---- | 05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, J.U.; Patil, P.N.; Pawar, N.S. Synthesis and Antibacterial Activity of Thymyl Ethers. Chem. Proc. 2022, 8, 57. https://doi.org/10.3390/ecsoc-25-11747
Patil JU, Patil PN, Pawar NS. Synthesis and Antibacterial Activity of Thymyl Ethers. Chemistry Proceedings. 2022; 8(1):57. https://doi.org/10.3390/ecsoc-25-11747
Chicago/Turabian StylePatil, Jagdish U., Pramod Nagraj Patil, and Nilesh S. Pawar. 2022. "Synthesis and Antibacterial Activity of Thymyl Ethers" Chemistry Proceedings 8, no. 1: 57. https://doi.org/10.3390/ecsoc-25-11747
APA StylePatil, J. U., Patil, P. N., & Pawar, N. S. (2022). Synthesis and Antibacterial Activity of Thymyl Ethers. Chemistry Proceedings, 8(1), 57. https://doi.org/10.3390/ecsoc-25-11747






