Abstract
In present work, one-pot synthesis of some new 2,4-disubstitued thiazolyl pyrazole derivatives was carried out. The reaction of different pyrazole 4-carbalaldehydes, thiosemicarbazides and α-haloketones in one pot afforded the target molecules. The synthesis was carried out via two methods: one conventional method, whereby pyrazole 4-carbaldehydes, thiosemicarbazides, and α-haloketones were refluxed in ethanol; and a second way, where the reaction mixture was ground at RT. The rate of the reaction, yield of the products, and purity of the products were compared for both methods. All of the synthesized compounds were tested for their antimicrobial activities. It was found that most of the compounds showed good-to-moderate antibacterial as well as antifungal activities.
1. Introduction
The importance of new biologically active molecules in the pharmaceutical industry has encouraged chemists to engage in their capable and fast synthesis, so as to provide useful benefits for society. Today, modern and fast technologies have motivated scientists and researchers to synthesize and develop new effective drug molecules. The synthesis and design of pyrazole and thiazole derivatives are of great interest due to their extensive applications in the pharmaceutical and agrochemical industries. The interest in the study of pyrazole chemistry is still ongoing due to its broad spectrum of biological activities, such as antibacterial [1,2,3,4,5,6], antiviral [7,8], antiproliferative, proapoptotic [9], antitumor [10], anti-inflammatory [11,12], and herbicidal activities [13]. Furthermore, thiazole heterocycles are a noteworthy class of heterocyclic compounds that are present in several important biologically dynamic drug molecules, such as the antiretroviral drug ritonavir, the antimicrobial drug sulfathiazole, the antineoplastic drug tiazofurin, and the antifungal drug abafungin [14]. Thiazole-containing heterocycles show various biological activities, such as antifungal [15], anticancer [16,17,18,19,20], and anti-HIV activity [21], as well as acting as a metabotropic glutamate receptor 1 (mGluR1) antagonist [22]. On the other hand, it has been observed that when thiazole is in combination with the pyrazole nucleus, it exhibits different biological activities, including antitubercular [23,24,25,26,27,28], anti-inflammatory, and antimicrobial effects [29,30], as well as acting as a protein synthase III (FabH) inhibitor [31].
All of these observations inspired us to design and synthesize new effective drug molecules containing thiazole and pyrazole nuclei together, and to assess their antibacterial and antifungal activities, expecting that these new moieties could be the effective heterocycle in the library of recognized drug molecules. Thus, in our study we synthesized a new derivative of heterocycles containing pyrazole and thiazole molecules in one component, which may show more effective biological activities.
In an extension of our work [32,33,34,35] on the preparation of new products with combinations of dissimilar heterocyclic moieties as possible antimicrobial agents, we report here the synthesis of some new pyrazole derivatives containing thiazole scaffolds. The intermediate pyrazole carbaldehydes 3a–d were synthesized by a known method from the literature [36,37]. A series of pyrazole-containing thiazole derivatives 4a–l (Scheme 1) were synthesized, and all of the synthesized compounds were screened for their antimicrobial activity.
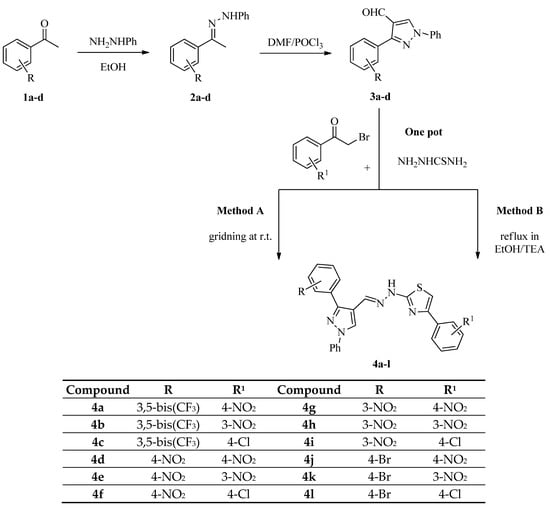
Scheme 1.
Synthetic route of 4a–l.
2. Results and Discussion
The structure of all of the synthesized compounds 4a–l was characterized by analysis of IR, 1H NMR, 13C NMR, mass, and elemental spectra. The IR bands at 3300–3340, 1545–1550, and 1620–1640 cm−1 showed the presence of NH, C=C, and C=N, respectively. In the 1H NMR spectra, a broad signal appeared at 12.1 ppm due to NH, a singlet appeared at 9.1–9.2 ppm due to a pyrazolyl proton, a singlet appeared at 7.68 ppm due to a thiazolyl proton, and a multiplet appeared at 7.4 to 8.3 ppm due to aromatic protons. The molecular ion peaks of all of the synthesized compounds were obtained from EI-MS, while the presence of M+2 peaks were characteristic for the compounds with chlorine, bromine, and sulfur atoms. Analogously, all other compounds were characterized by spectroscopic and analytical data, which are presented in the Experimental Section.
The grinding of aldehydes with thiosemicarbazides and α-haloketones was carried out at room temperature to afford the corresponding 2,4-disubstituted thiazole derivatives at a high yield (80–90%). In a typical procedure, pyrazole aldehydes react with thiosemicarbazides and α-haloketones to provide excellent yields of 2,4-disubstituted thiazoles after just a few minutes of grinding. To optimize the reaction conditions, the reaction between 3-(3,5-bis(trifluoromethyl) phenyl 1)-1-phenyl-1H-pyrazole-4-carbaldehyde thiosemicarbazide and 4-chloro phenacyl bromide was chosen as a model reaction as shown in Table 1. The reaction was completed after grinding for 4 min, and afforded a 2,4-disubstituted thiazole derivative with 85% yield. After optimizing the conditions, we next examined the scope and generality of this method using different pyrazole 4-carbaldehydes. It was observed that all reactions were completed within 5–10 min by grinding without any catalyst or solvent at ambient temperature. However, highly efficient grinding was required for the success of these reactions. When attempts were made to carry out the synthesis of thiazole derivatives by conventional methods in ethanol under reflux temperature, it required more time, and the yield of the products was in the range of 60–70% (Table 1). In general, reactions under solvent-free conditions were clean, rapid, and afforded higher yields than those obtained via conventional methods in ethanol.

Table 1.
Table showing the differences between the conventional and solvent-free methods.
3. Biological Results and Discussion
All of the synthesized compounds were screened for their antibacterial and antifungal activities, and the results are shown in Table 2. It was found that most of the compounds showed good-to-moderate activity against both Gram-positive and Gram-negative bacteria. It was noted that the substituent R on the phenyl ring does not affect the biological activity to a large extent, but the substituent R1 was found to play important role in determining the biological activity. It was observed that when R1 was a strong electron-withdrawing compound similar to NO2 (i.e., compounds 4a, 4d, 4g, and 4j), it showed enhancements in antifungal as well as antibacterial activities, as compared to compounds 4c, 4f, 4i, and 4l, where the substituent R1 was 4-Cl. The derivatives in which the R1 group was at position 3 (i.e., compounds 4b, 4e, 4h, and 4k) showed less antimicrobial activities.

Table 2.
Antimicrobial screening of synthesized compounds 4a–l.
4. Experimental Section
4.1. General Procedure for the Synthesis of Phenyl Hydrazone Derivatives 2a–d
A mixture of substituted acetophenones 1a–d (1 mol), phenyl hydrazine (1 mol), and acetic acid (1 mL) in ethanol (20 mL) was refluxed for 30 min. After the completion of the reaction, as monitored via TLC, the reaction mixture was cooled at room temperature. The product was filtered, washed with water, dried, and recrystallized from ethanol. Physical data of compound 2a–d is mentioned in Table 3.

Table 3.
Physical data of compounds 2a–d.
4.2. General Procedure for the Synthesis of 1-Phenyl-3-(substituted -phenyl)-1H-pyrazole-4-carbaldehydes 3a–d
To a well-stirred and cooled (0 °C) DMF solution (12 mL), POCl3 (6 mL) was added dropwise for 1 h. After complete addition of POCl3, the reaction mixture was further stirred at 0 °C for 1 h. To this well-stirred and cooled reaction mixture, a solution of 2a–d (1 mol) in anhydrous DMF (10 mL) was added dropwise for one hour; after complete addition, the reaction mixture was heated at 65–70 °C for 2 h. The reaction mixture was poured onto crushed ice and left overnight in a refrigerator, during which time the product separated out as a solid mass. The product was filtered, washed with Na2CO3 (5%, 30 mL) and water, and recrystallized from the DMF–ethanol mixture. Physical data of compound 3a–d is mentioned in Table 4.

Table 4.
Physical data of compounds 3a–d.
4.3. General Procedure for the Synthesis of 2,4-Disubstitutde Thiazole Derivatives 4a–l
4.3.1. Method A
A mixture of pyrazole aldehyde (1 mmol), thiosemicarbazide (1 mmol), and α-haloketone (1 mmol) was ground thoroughly with a pestle and mortar at room temperature for 5–10 min. The progress of the reaction was monitored by TLC (ethyl acetate/hexanes 3:7). After completion of the reaction, the mixture was washed with water and recrystallized from ethanol to yield the pure product.
4.3.2. Method B
A mixture of pyrazole aldehyde (1 mmol), thiosemicarbazide (1 mmol), and α-haloketone (1 mmol) in ethanol was refluxed for 3 h. The reaction mixture was cooled at room temperature and poured onto crushed ice. The separated solid was filtered, washed with ice-cold water, and purified by column chromatography (Ethyl acetate/hexanes 2:8).
5. Spectral Data
1-((3-(3,5-bis(trifluoromethyl)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(4-nitrophenyl)thiazol-2-yl)hydrazine (4a) m.p.: 236–238 °C; IR (KBr, cm−1): 3340 (NH), 1545 (C=C), 1620 (C=N); 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9.1 (s, 1H, pyrazolyl-H), 7.68 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 7.4–7.8 (m, 5H, Ar-H, Phenyl ring), 8.4 (s, 2H, Ar-H), 8.3 (s, 1H Ar-H), 8.2 (d, J = 7.9 Hz, 2H), 8.3 (d, J = 7.9 Hz, 2H); 13C NMR (75 MHz, CDCl3): δ 168.1, 150.1, 148.5, 146.4, 140.5, 137.7, 109.1, 116.0, 119.2 (2C), 130.1 (2C), 126.3, 139.5, 126.6 (2C), 131.8 (2C), 130.7, 128.7, 129.5 (2C), [133.5, 133.9, 134.4, 134.8 (q, J = 34.5 Hz, 2C)], 127.8, [124.1, 120.5, 116.8, 113.2 (q, J = 272 Hz, 2C)]; MS (EI, 70 eV): m/z (%): 602 (M+, 100); Analysis calculated for C27H16F6N6O2S: C, 53.82; H, 2.68; N, 13.95; found: C, 53.43; H, 2.32; N, 14.15.
1-((3-(3,5-bis(trifluoromethyl)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(3-nitrophenyl)thiazol-2-yl)hydrazine (4b) m.p.: 230–235 °C; IR (KBr, cm−1): 3350 (NH), 1550 (C=C), 1625 (C=N); 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9.1 (s, 1H, pyrazolyl-H), 7.7 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 7.4–7.8 (m, 5H, Ar-H, Phenyl ring), 8.4 (s, 2H, Ar-H), 8.3 (s, 1H Ar-H), 7.7–8.6 (m, 4H, m-NO2 phenyl protons); MS (EI, 70 eV): m/z (%): 602 (M+, 100); Analysis calculated for C27H16F6N6O2S: C, 53.82; H, 2.68; N, 13.95; found: C, 53.55; H, 2.38; N, 14.25.
1-((3-(3,5-bis(trifluoromethyl)phenyl)-1-phenyl-1H-pyrazol-4-yl) methylene)-2-(4-(4-chlorophenyl)thiazol-2-yl)hydrazine (4c) m.p.: 238–240 °C; IR (KBr, cm−1): 3340 (NH), 1545 (C=C), 1620 (C=N); 1H NMR (300 MHz, DMSO-d6): δ 12.0 (bs, 1H, NH), 9 (s, 1H, pyrazolyl-H), 7.7 (s, 1H, thiazolyl-H), 8.3 (s, 1H, CH=N), 7.4–7.8 (m, 5H, Ar-H, Phenyl ring), 8.4 (s, 2H, Ar-H), 8.3 (s, 1H Ar-H), 7.9 (d, J = 8.3 Hz, 2H), 8.2 (d, J = 8.3 Hz, 2H); 13C NMR (75 MHz, DMSO-d6): δ 168.0, 150.0, 148.4, 146.5, 140.1, 136.0, 109.0, 116.0, 119.3 (2C), 129.0 (2C), 126.4, 139.4, 125.6 (2C), 130.1 (2C), 129.4, 128.7, 129.6 (2C), [133.5, 133.9, 134.4, 134.8 (q, J = 34.5 Hz, 2C)], 128.0, [124.1, 120.5, 116.8, 113.2 (q, J = 272 Hz, 2C)]; MS (EI, 70 eV): m/z (%): 591 (M+, 100); Analysis calculated for C27H16ClF6N5S: C, 54.78; H, 2.72; N, 11.83; found: C, 54.57; H, 2.48; N, 12.04.
1-((3-(4-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(4-nitrophenyl)thiazol-2-yl)hydrazine (4d) m.p.: 213–216 °C; 1H NMR (300 MHz, DMSO-d6): δ 12.0 (bs, 1H, NH), 9.1 (s, 1H, pyrazolyl-H), 7.7 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 7.4–7.8 (m, 5H, Ar-H, Phenyl ring), 8.2 (d, J = 7.9 Hz, 2H), 7.9 (d, J = 7.9 Hz, 2H), 8.3 (d, J = 8.1 Hz, 2H), 8.1 (d, J = 8.1 Hz, 2H); 13C NMR(75 MHz, DMSO-d6): δ 169.2, 150.0, 149.1, 147.0, 141.4, 138.0, 136.1, 109.4, 118.0, 120.0 (2C), 130.1 (2C), 128.0, 125.5 (2C), 129.0 (2C), 129.1 (2C), 136.0 (2C), 136.5 (2C), 136.2, 125.4 (2C); MS (EI, 70 eV): m/z (%): 511 (M+, 100); Analysis calculated for C25H17N7O4S: C, 58.70; H, 3.35; N, 19.17; found: C, 58.58; H, 4.11; N, 19.63.
1-((3-(4-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(3-nitrophenyl) thiazol-2-yl)hydrazine (4e) m.p.: 222–225 °C; IR (KBr, cm−1): 3350 (NH), 1560 (C=C), 1600 (C=N), 1350, 1540 (NO2); 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9.0 (s, 1H, pyrazolyl-H), 7.5 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 7.9–8.4 (m, 4H, m-NO2), 8.4 (d, J = 7.9 Hz, 2H), 8.2 (d, J = 7.9 Hz, 2H), 7.5–7.7 (m, 5H, Ar-H phenyl); MS (EI, 70 eV): m/z (%): 511 (M+, 100); Analysis calculated for C25H17N7O4S: C, 58.70; H, 3.35; N, 19.17; found: C, 58.35; H, 3.61; N, 19.52.
1-((3-(4-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(4-chlorophenyl) thiazol-2-yl)hydrazine (4f) m.p.: 234–239 °C; IR (KBr, cm−1): 3300 (NH), 1555 (C=C), 1615 (C=N), 3322, 3022 (Ar-H), 1355, 1550 (NO2), 965; 1H NMR (300 MHz, DMSO-d6) δ 12.0 (bs, 1H, NH), 9.1 (s, 1H, pyrazolyl-H), 7.6 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 7.4–7.8 (m, 5H, Ar-H, Phenyl ring), 7.9 (d, J = 8.2 Hz, 2H), 8.1 (d, J = 8.2Hz, 2H), 8.3 (d, J = 8 Hz, 2H), 8.2 (d, J = 8 Hz, 2H); MS (EI, 70 eV): m/z (%): 500 (M+, 100); Analysis calculated for C25H17ClN6O2S: C, 59.94; H, 3.42; N, 16.78; found: C, 60.11; H, 3.62; N, 16.50.
1-((3-(3-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(4-nitrophenyl)thiazol-2-yl)hydrazine (4g) m.p.: 230–235 °C; IR (KBr, cm−1): 3350 (NH), 1550 (C=C), 1620 (C=N), 3315, (Ar-H), 1330, 1540 (NO2) 950; 1H NMR (300 MHz, DMSO-d6): δ 12.0 (bs, 1H, NH), 9.1 (s, 1H, pyrazolyl-H), 7.4 (s, 1H, thiazolyl-H), 8.3 (s, 1H, CH=N), 7.8–8.3 (m, 4H, m-NO2), 8.2 (d, J = 8.2 Hz, 2H), 8.3 (d, J = 8.2 Hz, 2H), 7.5–7.8 (m, 5H, Ar-H phenyl); MS (EI, 70 eV): m/z (%): 511 (M+, 100); Analysis calculated for C25H17N7O4S: C, 58.70; H, 3.35; N, 19.17; found: C, 58.54; H, 3.60; N, 19.01.
1-((3-(3-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-(4-(3-nitrophenyl) thiazol-2- yl)hydrazine (4h) m.p.: 224–228 °C; 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9.1(s, 1H, pyrazolyl-H), 7.3 (s, 1H, thiazolyl-H), 8.2 (s, 1H, CH=N), 7.9–8.6(m, 8H, m-NO2 phenyl rings), 7.4–7.8(m, 5H, Ar-H phenyl); 13C NMR(75 MHz, CDCl3): δ 149.5, 140.7, 118.7(2C), 129.7(2C), 126.4, 135.0, 117.0, 146.3, 168.0, 146.5, 108.8, 133.9(2C), 132.8(2C), 130.4(2C), 120.9(2C), 148.9(2C), 122.4(2C); MS (EI, 70 eV): m/z (%): Analysis calculated for C25H17N7O4S: C, 58.70; H, 3.35; N, 19.17; found: C, 58.64; H, 3.50; N, 19.08.
1-((3-(3-Nitro-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-(4-chloro-phenyl) thiazol-2-yl)hydrazine (4i) m.p.: 220–225 °C; IR (KBr, cm−1): 3350 (NH), 1545 (C=C), 1622 (C=N), 3320 (Ar-H), 1345, 1545 (NO2) 950; 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9 (s, 1H, pyrazolyl-H), 7.5 (s, 1H, thiazolyl-H), 8.6 (s, 1H, CH=N) 7.9–8.3 (m, 4H, m-NO2), 8.0 (d, J = 8.2 Hz, 2H), 7.9 (d, J = 8.2Hz, 2H), 7.4–7.7 (m, 5H, Ar-H phenyl); 13C NMR (75 MHz, CDCl3): δ 134.0, 129.5 (2C), 129.0 (2C), 131.1, 149.6, 140.7, 118.8 (2C), 129.7 (2C), 126.4, 135.1, 117.0, 146.3, 168.5, 108.5, 148.6, 134.0, 132.0, 130.5, 121.0, 140.5, 122.5; MS (EI, 70 eV): m/z (%): 500 (M+, 100); Analysis calculated for C25H17ClN6O2S: C, 59.94; H, 3.42; N, 16.78; found: C, 60.10; H, 3.12 ; N, 16.45.
1-((3-(4-Bromo-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-(4-Nitro-phenyl)thiazol-2-yl)hydrazine (4j) m.p.: 230–233 °C; IR (KBr, cm−1): 3355 (NH), 1555 (C=C), 1610 (C=N), 3320, (Ar-H), 950; 1H NMR (300 MHz, DMSO-d6): δ 12.0 (bs, 1H, NH), 8.9 (s, 1H, pyrazolyl-H), 7.6 (s, 1H, thiazolyl-H), 8.0 (s, 1H, CH=N), 7.8 (d, J = 8.3 Hz, 2H), 8.2 (d, J = 8.3 Hz, 2H), 7.9 (d, J = 8.1 Hz, 2H), 8.3 (d, J = 8.1 Hz, 2H), 7.3–7.6 (m, 5H, Ar-H); 13C NMR (75 MHz, DMSO-d6): δ 168.6, 150.0, 148, 146.2, 140.7, 138.0, 135.0, 109.1, 117.2, 119.0 (2C), 129.9 (2C), 126.0, 125.5 (2C), 129.6(2C), 136.4, 128.8, 124.0 (2C), 127.0 (2C), 132.2; MS (EI, 70 eV): m/z (%): 544 (M+, 100).
1-((3-(4-Bromo-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-(3-Nitro-phenyl)thiazol-2-yl)hydrazine (4k) m.p.: 212–215 °C; IR (KBr, cm−1): 3350 (NH), 1550 (C=C), 1620 (C=N), 3315, (Ar-H), 1330, 1540 (NO2) 950; 1H NMR (300 MHz, DMSO-d6): δ 12.1 (bs, 1H, NH), 9.0 (s, 1H, pyrazolyl-H), 7.5 (s, 1H, thiazolyl-H), 8.4 (s, 1H, CH=N), 8.3–8.5 (m, 4H, m-NO2 phenyl ring), 7.9 (d, J = 8.3 Hz, 2H), 8.1 (d, J = 8.3 Hz, 2H), 7.4–7.6 (m, 5H, Ar-H phenyl); 13C NMR (75 MHz, CDCl3): δ 122.8, 131.5 (2C), 129.1 (2C), 131.9, 149.4, 140.7, 118.7 (2C), 129.7 (2C), 126.4, 135.0, 117.2, 146.3, 167.9, 108.5, 148.5, 134.0, 132.0, 130.2, 121.1, 139.9, 122.5; MS (EI, 70 eV): m/z (%): 544 (M+, 100); Analysis calculated for C25H17BrN6O2S: C, 55.05; H, 3.14; N, 15.41; found: C, 55.25; H, 3.35; N, 15.15.
1-((3-(4-Bromo-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-(4-chloro-phenyl)thiazol-2-yl)hydrazine (4l) m.p.: 235–237 °C; IR (KBr, cm−1): 3350 (NH), 1550 (C=C), 1610 (C=N), 3323, 3021 (Ar-H), 960, 850, 720; 1H NMR (300 MHz, DMSO-d6): δ 12.0 (bs, 1H, NH), 8.9 (s, 1H, pyrazolyl-H), 7.7 (s, 1H, thiazolyl-H), 8.1 (s, 1H, CH=N), 7.9 (d, J = 8.2 Hz, 2H), 8.1 (d, J = 8.2 Hz, 2H), 7.7 (d, J = 8.3 Hz, 2H), 8.2 (d, J = 8.3 Hz, 2H), 7.4–7.6 (m, 5H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 168.5, 149.6, 148.6, 146.3, 140.7, 139.0, 135.1, 108.5, 117.0, 118.8 (2C), 129.7 (2C), 126.4, 125.3 (2C), 131.5 (2C), 130.6, 128.7, 124.2 (2C), 127.1 (2C), 132.0; MS (EI, 70 eV): m/z (%): 533 (M+,100); Analysis calculated for C25H17BrClN5S: C, 56.14; H, 3.20; N, 13.09; found: C, 56.54; H, 3.64; N, 12.89.
6. Conclusions
In conclusion, we developed a new series of pyrazole derivatives containing thiazole heterocyclic rings. The in vitro antimicrobial assay showed that most of the synthesized compounds showed good activity as compared to standard drugs. From the biological activity report it can be concluded that pyrazole and thiazole heterocyclic rings play an important role in determining the biological activity. It was therefore of interest to explore these azoles for additional modification in order to design new heterocycles for use as potent drugs.
Author Contributions
Designing of the molecules, S.V.P.; Literature and spectral analysis, M.B.S.; Synthesis of compounds, D.R.N.; Paper writing and editing, S.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The author is thankful to the G. E. Society’s HPT Arts and RYK Science College, Nashik, for providing laboratory facilities. The author also thanks BCUD, Pune University, and UGC, New Delhi for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Liu, X.H.; Cui, P.; Song, B.A.; Bhadury, P.S.; Zhu, H.L.; Wang, S.F. Synthesis, structure and antibacterial activity of novel 1-(5-substituted-3-substituted-4,5-dihydro pyrazol-1-yl)ethanone oxime ester derivatives. Bioorg. Med. Chem. 2008, 16, 4075–4082. [Google Scholar] [CrossRef]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Salhi, S.; Radi, A. Synthesis and Preliminary Biological Activity of Some New Pyrazole Derivatives as Acyclonucleoside Analogues. Lett. Drug Des. Discov. 2010, 7, 27–30. [Google Scholar] [CrossRef]
- Sridhar, R.; Perumal, P.J.; Etti, S.; Shanmugam, G.; Ponnuswamy, M.N.; Prabavathy, V.R.; Mathivanan, N. Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg. Med. Chem. Lett. 2004, 14, 6035–6040. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, R.; Sehrawat, R. Synthesis and antibacterial activity of some new 2,3-dimethoxy-3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl) chroma nones. Eur. J. Med. Chem. 2008, 44, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.M.; Abdallah, M.A.; Gomha, S.M.; Kazem, M.S.H. Synthesis and antimicrobial activity of novel azolopyrimidines and pyrido-triazolo-pyrimidinones incorporating pyrazole moiety. J. Heterocycl. Chem. 2017, 54, 3447–3457. [Google Scholar] [CrossRef]
- Ouyang, G.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Gomha, S.M.; Abdelaziz, M.R.; Serag, N. Synthesis and antiviral evaluation of some novel thiazoles and 1,3-thiazines substituted with pyrazole moiety against rabies virus. Turk. J. Chem. 2016, 40, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Anzaldi, M.; Maccio, C.; Mazzei, M.; Bertolotto, M.; Ottonello, L.; Dallegri, F.; Balbi, A. Antiproliferative and proapoptotic activities of a new class of pyrazole derivatives in HL-60 cells. Chem. Biodivers. 2009, 6, 1674–1687. [Google Scholar] [CrossRef]
- El-Shafei, A.; Fadda, A.A.; Khalil, A.M.; Ameen, T.; Badria, F.A. Synthesis, antitumor evaluation, molecular modeling and quantitative structure-activity relationship (QSAR) of some novel arylazopyrazolodiazine and triazine analogs. Bioorg. Med. Chem. 2009, 17, 5096–5105. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Ashour, H.M.A.; Ghany, Y.S.A.; El-Din, A.; Bekhit, A.; Baraka, A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Dadiboyena, S.; Valente, E.J.; Hamme, A.T. A novel synthesis of 1,3,5-trisubstituted pyrazoles through a spiro-pyrazoline intermediate via a tandem 1,3-dipolar cycloaddition/elimination. Tetrahedron Lett. 2009, 50, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Noriaki, K.; Satoru, F. Synthesis and Herbicidal Activity of 1,5-Diarylpyrazole Derivatives. Chem. Pharm. Bull. 1999, 47, 857–859. [Google Scholar]
- Siddiqui, N.; Arshad, M.F.; Ahsan, W. Thiazoles: A valuable insight into the recent advances and biological activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar]
- Vasu, N.; Goud, B.B.; Kumari, Y.B.; Rajitha, B. Design, synthesis and biological evaluation of some novel benimidazole based thiazolyl amines. Rasayan J. Chem. 2013, 6, 201–206. [Google Scholar]
- Singh, N.; Bhati, S.K.; Kumar, A. Thiazolyl/oxazolyl formazanyl indoles as potent anti-inflammatory agents. Eur. J. Med. Chem. 2008, 43, 2597–2609. [Google Scholar] [CrossRef]
- Luzina, E.L.; Popov, A.V. Synthesis and anticancer activity of N-bis(trifluoromethyl)alkyl-N′-thiazolyl and N-bis(trifluoromethyl)alkyl-N’-benzothiazolyl ureas. Eur. J. Med. Chem. 2009, 44, 4944–4953. [Google Scholar] [CrossRef]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo [3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009, 44, 3627–3636. [Google Scholar] [CrossRef]
- Rawal, R.K.; Tripathi, R.; Katti, S.B.; Pannecouque, C.; De, C.E. Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIVagents. Eur. J. Med. Chem. 2008, 43, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Nagatomi, Y.; Hirata, Y.; Ito, S.; Suzuki, G.; Kimura, T.; Maehara, S.; Hikichi, H.; Satow, A.; Hata, M.; et al. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 2009, 19, 5464–5468. [Google Scholar] [CrossRef]
- Azzali, E.; Machado, D.; Kaushik, A.; Vacondio, F.; Flisi, S.; Cabassi, C.S.; Lamichhane, G.; Viveiros, M.; Costantino, G.; Pieroni, M. Substituted N-phenyl-5-(2-(phenylamino)thiazol-4-yl)isoxazole-3-carboxamides are valuable antitubercular candidates that evade innate efflux machinery. J. Med. Chem. 2017, 60, 7108–7122. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Surana, S.; Jain, P.; Patel, H.M. Mycobacterium Tuberculosis (MTB) GyrB inhibitors: An attractive approach for developing novel drugs against TB. Eur. J. Med. Chem. 2016, 124, 160–185. [Google Scholar] [CrossRef]
- Vaarla, K.; Kesharwani, R.K.; Santosh, K.; Vedula, R.R.; Kotamraju, S.; Toopurani, M.K. Synthesis, biological activity evaluation and molecular docking studies of novel coumarin substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes. Bioorg. Med. Chem. Lett. 2015, 25, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, K.M.; Cui, H.E.; Yang, Y.S.; Luo, Y.; Xing, M.; Qiu, X.Y.; Bai, L.F.; Zhu, H.L. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives containing benzodioxole as potential anticancer agents. Bioorg. Med. Chem. 2013, 21, 448–455. [Google Scholar] [CrossRef]
- Bridges, A.J. The rationale and strategy used to develop a series of highly potent, irreversible, inhibitors of the epidermal growth factor receptor family of tyrosine kinases. Curr. Med. Chem. 1999, 6, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.C.; Li, D.D.; Li, Q.S.; Lu, X.; Xiao, Z.P.; Zhu, H.L. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg. Med. Chem. Lett. 2011, 21, 5374–5377. [Google Scholar] [CrossRef] [PubMed]
- Khloya, P.; Kumar, S.; Kaushik, P.; Surain, P.; Kaushik, D.; Sharma, P.K. Synthesis and biological evaluation of pyrazolylthiazole carboxylic acids as potent anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1177–1181. [Google Scholar] [CrossRef]
- Takate, S.J.; Shinde, A.D.; Karale, B.K.; Akolkar, H.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Thiazolyl-pyrazole derivatives as potential antimycobacterial agents. Bioorg. Med. Chem. Lett. 2019, 29, 1199–1202. [Google Scholar] [CrossRef]
- Yang, Y.S.; Zhang, F.; Gao, C.; Zhang, Y.B.; Wang, X.L.; Tang, J.F.; Sun, J.; Gong, H.B.; Zhu, H.L. Discovery and modification of sulfur-containing heterocyclic pyrazoline derivatives as potential novel class of β-ketoacyl-acyl carrier protein synthase III (FabH) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4619–4624. [Google Scholar] [CrossRef]
- Bobade, V.; Gaikwad, N.D.; Patil, S.V. Synthesis and biological evaluation of some novel thiazole substituted benzotriazole derivatives. Bioorg. Med. Chem. 2012, 22, 3449–3454. [Google Scholar]
- Nalawade, V.; Patil, S.V.; Bobade, V.D.; Mhaske, P.C. Synthesis of new thiazolyl-pyrazolyl-1,2,3-triazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2019, 179, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, V.; Patil, S.V.; Bobade, V.D.; Mhaske, P.C. Synthesis, Characterization, and Antimicrobial Screening of 4″-methyl-2,2″-diaryl-4,2′:4,5″-terthiazole Derivatives. J. Heterocycl. Chem. 2018, 55, 1366–1374. [Google Scholar] [CrossRef]
- Patil, S.V.; Bondarde, M. An efficient one pot synthesis and antimicrobial activity of some new thiazole substituted pyrazole derivatives. IJCPS 2018, 7, 268–274. [Google Scholar]
- Bakr, F.; Abdel, W.; Rizk, E.K.; Farahat, A.A. Pyrazole-3(4)-carbaldehyde: Synthesis, reactions and biological activity. ARKIVOC Online J. Org. Chem. 2011, 2011, 196–245. [Google Scholar]
- Ilya, V.; Taydakov, S.S.; Tatiana, Y. Improved Synthesis of 1H-Pyrazole-4-carbaldehyde. Synth. Commun. 2011, 41, 2430–2434. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).