Patentability of Biopolymer-Based Hydrogels †
Abstract
:1. Introduction
2. Resources and Research Methods
3. Analysis of the Patentability of Biopolymer-Based Hydrogels
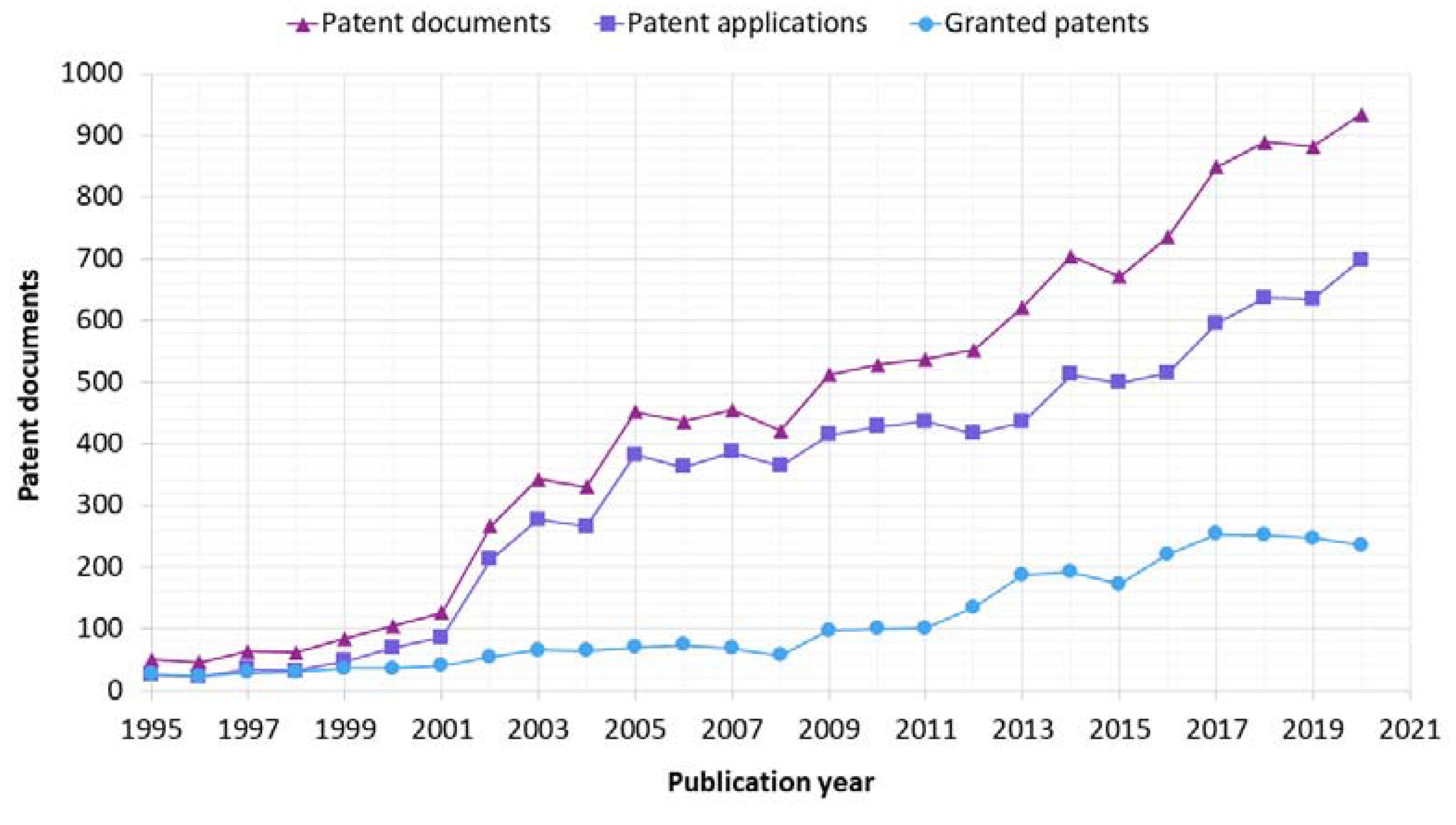
3.1. Publication Year
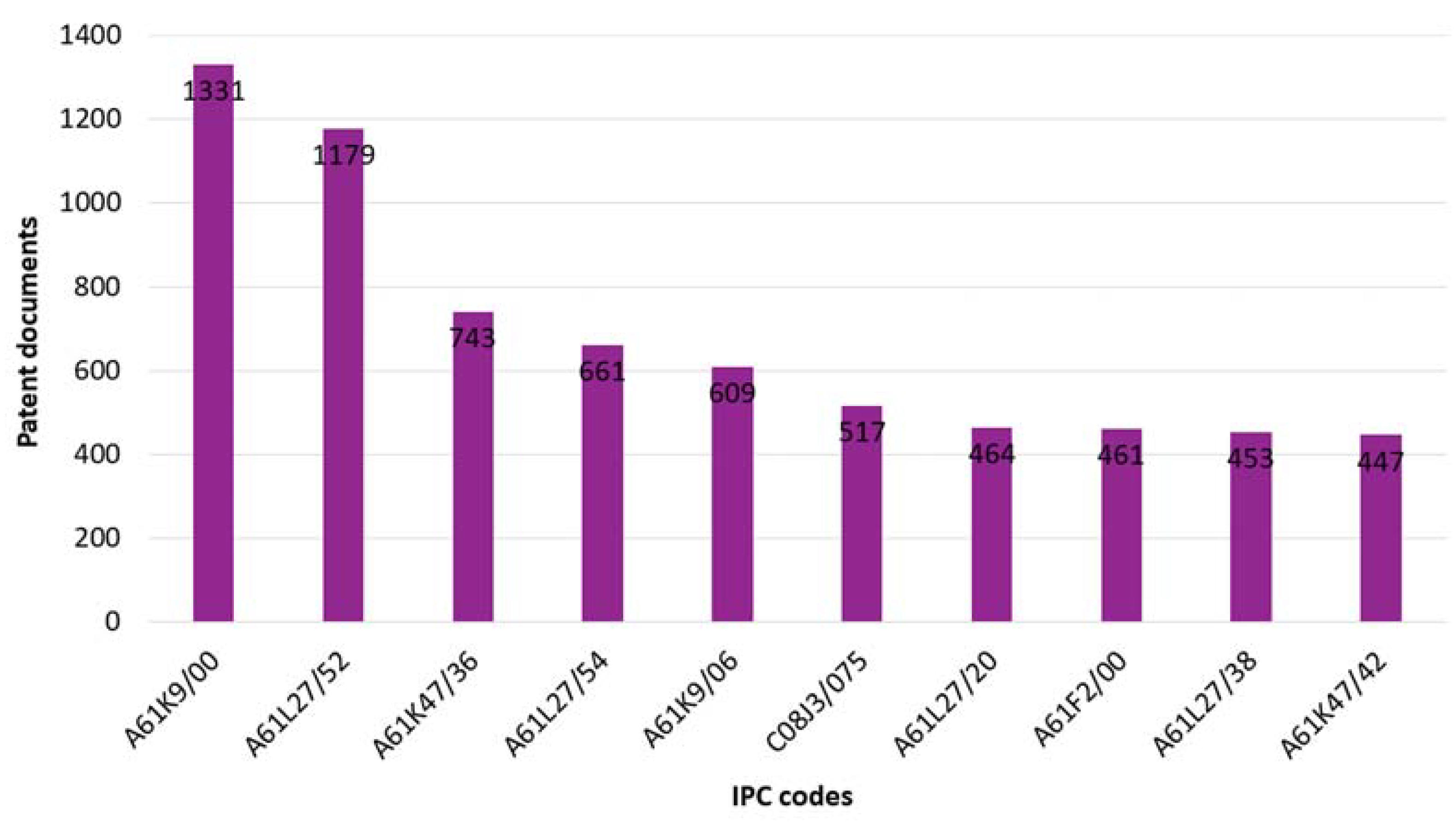
3.2. International Patent Classification
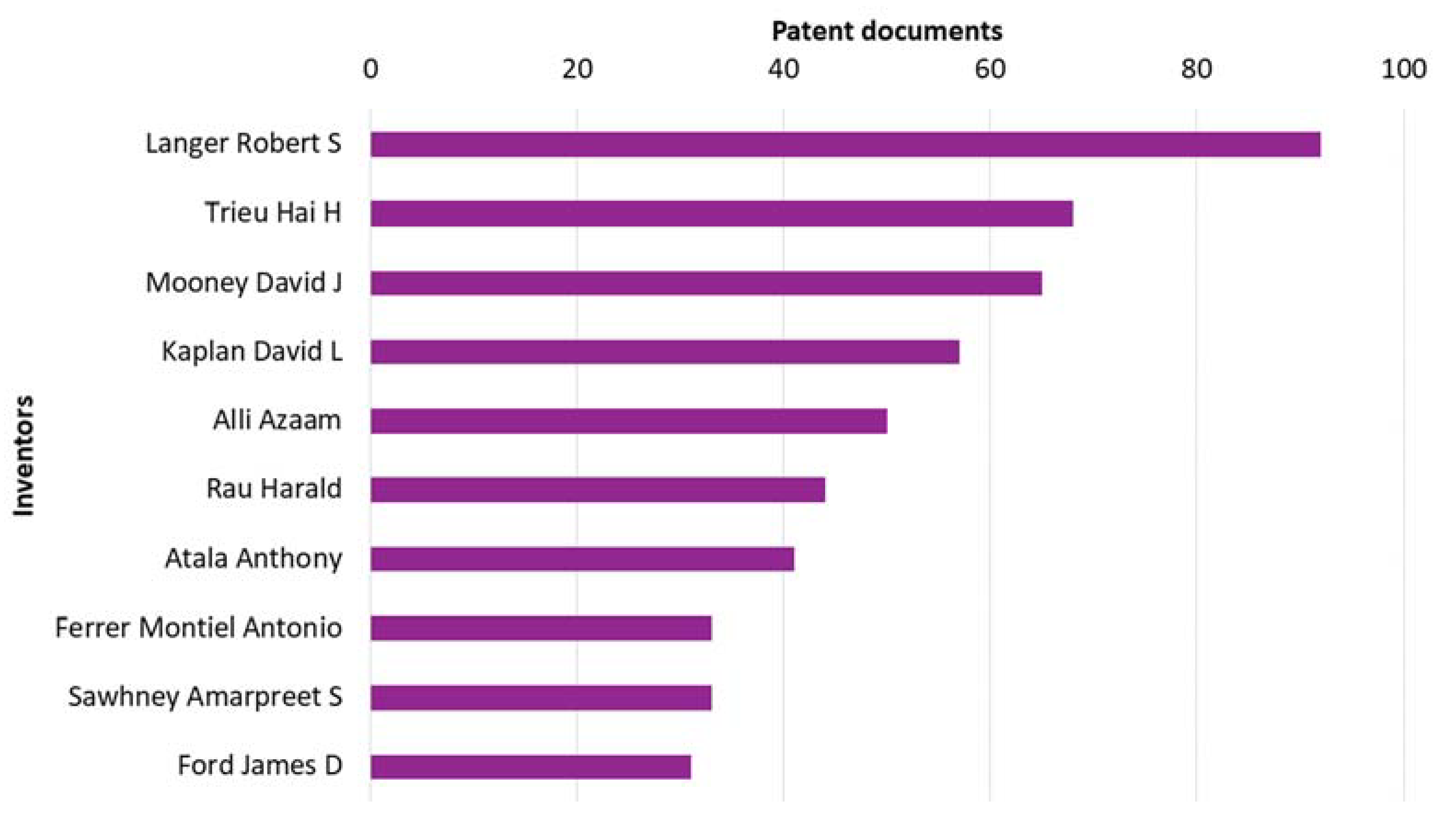
3.3. Inventors
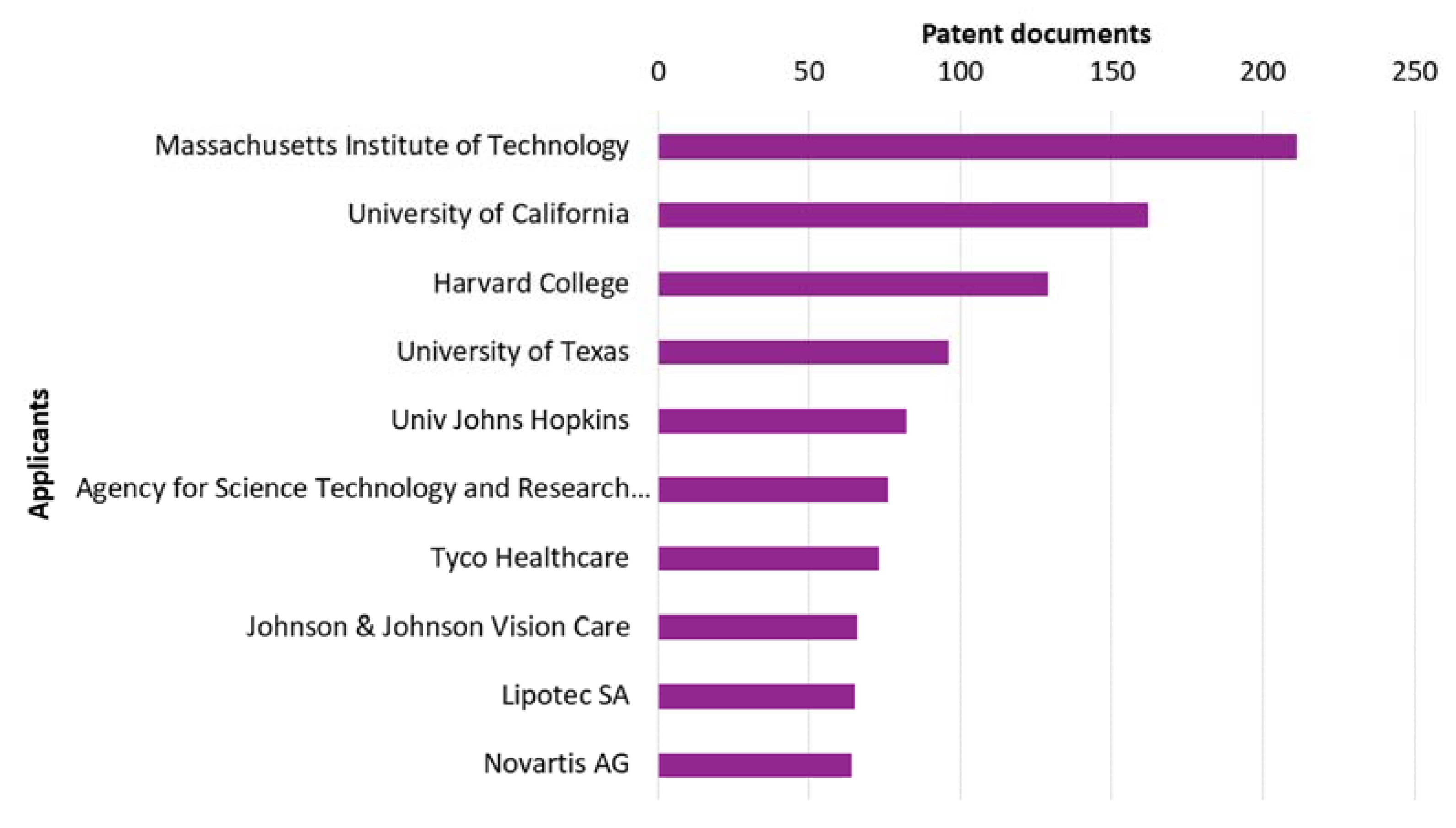
3.4. Applicants
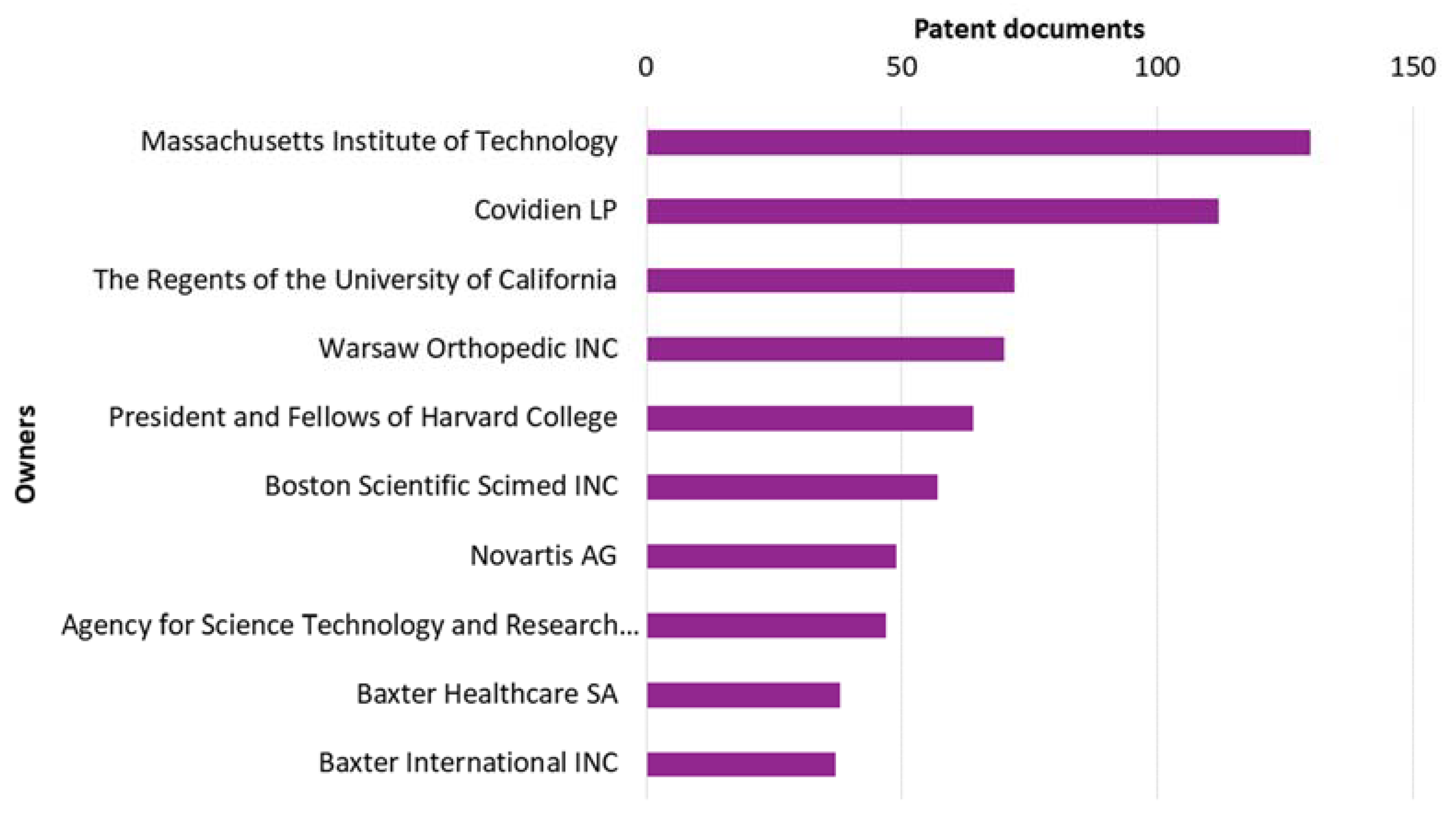
3.5. Owners
3.6. Jurisdiction
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabasci, S. Biobased plastics. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Oxford, UK, 2020; pp. 67–96. [Google Scholar]
- Ruso, J.M.; Messina, P.V. Preface. In Biopolymers for Medical Applications, 1st ed.; Ruso, J.M., Messina, P.V., Eds.; CRC Press: Boca Raton, FL, USA, 2016; p. 372. [Google Scholar]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. Part A 2010, 94A, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin templated polypeptide co-cross-linked hydrogel for bone regeneration. Adv. Healthc. Mater. 2020, 9, 1901239. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, H.; Moon, S.; Na, K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J. Biosci. Bioeng. 2009, 108, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Tassin, J.F.; Turczyn, R.; Axelos, M.A.; Weiss, P. Gelation studies of a cellulose-based biohydrogel: The influence of pH, temperature and sterilization. Acta Biomater. 2009, 5, 3423–3432. [Google Scholar] [CrossRef] [Green Version]
- Tapan Kumar, G.; Deepa, T.; Amit, A.; Ajazuddin, A.; Hemant, B.; Dulal Krishna, T. Alginate based Hydrogel as a Potential Biopolymeric Carrier for Drug Delivery and Cell Delivery Systems: Present Status and Applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef]
- Fatimi, A. Chitosan-based embolizing hydrogel for the treatment of endoleaks after endovascular aneurysm repair. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 107–114. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.F.; Quillard, S.; Axelos, M.A.; Weiss, P. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials 2008, 29, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horkay, F. Polyelectrolyte Gels: A Unique Class of Soft Materials. Gels 2021, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Julius, B. A Process for Converting Acids into Stable Colloidal Systems. GB Patent 457769 A, 30 November 1936. [Google Scholar]
- Ma, S.; Yu, B.; Pei, X.; Zhou, F. Structural hydrogels. Polymer 2016, 98, 516–535. [Google Scholar] [CrossRef]
- World Intellectual Property Organization. Patentscope. Available online: https://patentscope.wipo.int (accessed on 2 September 2021).
- World Intellectual Property Organization. Patentscope Fields Definition. Available online: https://patentscope.wipo.int/search/en/help/fieldsHelp.jsf (accessed on 2 September 2021).
- Cambia Institute. The Lens Patent Data Set. Version 8.0.14. Available online: https://www.lens.org (accessed on 2 September 2021).
- European Patent Office. Espacenet Glossary. Version 1.24.1. Available online: https://worldwide.espacenet.com/patent (accessed on 2 September 2021).
- World Intellectual Property Organization. IPC Publication. IPCPUB v8.5. Available online: https://www.wipo.int/classifications/ipc/ipcpub (accessed on 2 September 2021).
- World Intellectual Property Organization. Guide to the International Patent Classification (IPC). Available online: https://www.wipo.int/edocs/pubdocs/en/wipo_guide_ipc_2020.pdf (accessed on 2 September 2021).
- World Intellectual Property Organization. What Is Intellectual Property? Frequently Asked Questions: Patents. Available online: https://www.wipo.int/patents/en/faq_patents.html (accessed on 2 September 2021).
| Hydrogel | Properties | Application |
|---|---|---|
| Collagen | Low immune response, good substrate for cell adhesion, chemotactic. | Corneal substitutes; Wound healing; Bone tissue engineering. |
| Easily remodeled and degraded by cells. | ||
| Chemical crosslinking decreases degradation and improves long-term mechanical properties. | ||
| Gelatin | Irreversibly hydrolyzed form of collagen. | Drug and cell delivery; Cell encapsulation; Wound healing; Skin substitute; Nerve regeneration; Bone repair. |
| Presence of amino acidic sequences in the structure. | ||
| Water soluble, non-toxic, inexpensive, and non-immunogenic material. | ||
| Highly biocompatible and biodegradable in a physiological environment. | ||
| Fibrin | Stimulates cell migration, osteoconduction and vascularization. | Skin regeneration; Cardiac tissue engineering; Growth factors encapsulation. |
| Fibrinolytic inhibitors, such as aprotinin or aminocaproic acid, reduce in vitro degradation rates. | ||
| Silk | Low enzymatic degradation rate controlled by crystallinity and some concerns arise on potential cytotoxic effects. | Skin regeneration; Cardiac tissue engineering; Growth factors encapsulation. |
| Intrinsic mechanical properties. | ||
| Mechanics tailored by modifying concentration, crystallization, molecular weight, and scaffold size. | ||
| Alginate | Degradation through ionic exchange with surrounding media. | Microencapsulation of cells; Wound healing; Drug and cell delivery; Pulposous nucleus regeneration. |
| Variations in local mechanical properties controlled by concentration of divalent cation (e.g., calcium ions). | ||
| Hyaluronic Acid | Minimal immune response and chemotactic combined with the adequate agents. | Corneal wound healing; Bone and cartilage reparation; Spinal cord injury repair; Tumor models. |
| Osteo-inductive and angiogenesis in combination with growth factors. | ||
| Chitosan | Soluble only in acidic conditions and insoluble in neutral and basic conditions. | Wound dressing; Drug delivery systems; Skin regeneration; Cartilage tissue engineering; Blood vessels embolization. |
| Hemostatic stimulates osteo-conduction and wound healing. | ||
| Degradability and shape-ability to fit the defect site. | ||
| Cellulose | Non-toxic and non-irritant material. | Wound dressing and transdermal patches; Ophthalmic preparations; Cartilage tissue engineering. |
| Chemical crosslinking improves solubility and long-term mechanical properties. | ||
| 3D interconnected structure suitable for cell maintenance and differentiation. | ||
| Poor degradability. | ||
| Agarose | Slow degradation profile and the low mechanical properties. | Wound healing; Cell culture; Cartilage tissue engineering; Drug release. |
| 3D scaffolds exhibiting soft and flexible structure suitable for cell maintenance and differentiation. | ||
| Carrageenan | Thermally, pH, and cation concentration responsive material, inexpensive, and easy to manipulate. | Controlled drug release; Tissue engineering; Skin regeneration; wound healing; Cartilage scaffold. |
| Effectiveness in maintaining the proliferative and chondrogenic potential of encapsulated cells. |
| IPC | Description |
|---|---|
| A61K9/00 | Preparations for medical, dental, or toilet purposes. More specifically, medicinal preparations characterized by special physical forms. |
| A61L27/52 | Materials characterized by their function or physical properties, such as hydrogels or hydrocolloids. |
| A61K47/36 | Medicinal preparations characterized by the non-active ingredients used. More specifically, macromolecular organic or inorganic compounds, such as polysaccharides and derivatives thereof (e.g., gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin). |
| A61L27/54 | Materials characterized by their function or physical properties, such as biologically active materials (e.g., therapeutic substances). |
| A61K9/06 | Preparations for medical, dental, or toilet purposes. More specifically, medicinal preparations characterized by special physical forms, such as ointments. |
| C08J3/075 | Processes of treating or compounding macromolecular substances. More specifically, making solutions, dispersions, lattices, or gels in aqueous media, such as macromolecular gels, by other methods than by solution, emulsion, or suspension polymerization techniques. |
| A61L27/20 | Macromolecular materials for prostheses or for coating prostheses, such as polysaccharides. |
| A61F2/00 | Filters implantable into blood vessels and prostheses (i.e., artificial substitutes or replacements for parts of the body), such as stents, artificial nails, dental prostheses, artificial kidneys, and artificial hearts. |
| A61L27/38 | Materials for prostheses or for coating prostheses containing ingredients of undetermined constitution or reaction products thereof, such as animal cells. |
| A61K47/42 | Medicinal preparations characterized by the non-active ingredients used. More specifically, macromolecular organic or inorganic compounds, such as proteins and derivatives thereof (e.g., albumin, gelatin, oligopeptides or polyamino acids). |
| Jurisdiction | Patent Documents | Patent Contribution (%) |
|---|---|---|
| United States | 5865 | 49.65 |
| PCT 1 | 3266 | 27.65 |
| Europe 2 | 1412 | 11.95 |
| China | 681 | 5.77 |
| Canada | 166 | 1.41 |
| Japan | 137 | 1.16 |
| Republic of Korea | 137 | 1.16 |
| Australia | 85 | 0.72 |
| Russia | 43 | 0.36 |
| Mexico | 20 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimi, A. Patentability of Biopolymer-Based Hydrogels. Chem. Proc. 2022, 8, 39. https://doi.org/10.3390/ecsoc-25-11653
Fatimi A. Patentability of Biopolymer-Based Hydrogels. Chemistry Proceedings. 2022; 8(1):39. https://doi.org/10.3390/ecsoc-25-11653
Chicago/Turabian StyleFatimi, Ahmed. 2022. "Patentability of Biopolymer-Based Hydrogels" Chemistry Proceedings 8, no. 1: 39. https://doi.org/10.3390/ecsoc-25-11653
APA StyleFatimi, A. (2022). Patentability of Biopolymer-Based Hydrogels. Chemistry Proceedings, 8(1), 39. https://doi.org/10.3390/ecsoc-25-11653






