Abstract
An efficient method for the synthesis of a partially deuterated analogue of the natural neuritogenic alkynol, lembehyne B, has been developed for the first time, based on the use of a new reaction of Ti-catalyzed cross-cyclomagnesiation of O-containing 1,2-dienes and terminal aliphatic 1,2-dienes using EtMgBr in high yield. The introduction of two deuterium atoms is carried out at the stage of treatment of the formed in situ magnesacyclopentane with D2O.
1. Introduction
Acetylene alcohol lembehyne B, isolated in trace amounts from the Indonesian sea sponge Haliclona sp. [1], exhibits neuritogenic activity on Neuro-2A neuroblastoma cells [2], and is also an inducer of early apoptosis of Jurkat, HL-60, and K562 cell cultures [3].
Numerous studies carried out in recent years have shown that partially deuterated analogues of drugs have better pharmacokinetic characteristics, leading to changes in the mechanisms of biotransformation and a decrease in toxicity [4].
Earlier, at the Laboratory of Catalytic Synthesis of the Institute of Petrochemistry and Catalysis of the Ufa Federal Research Center of the Russian Academy of Sciences, a complete synthesis of natural lembehyne B was carried out for the first time [3], using the Ti-catalyzed cross-cyclomagnesiation of terminal allenes at the key stage of the reaction (the Dzhemilev reaction) [5,6,7,8,9,10,11,12,13,14,15,16]. Having obtained positive results of the study of the cytotoxicity of this alkynol, we obtained its deuterated analogue and studied its cytotoxicity in vitro.
2. Results and Discussion
At the first stage, the reaction of cross-cyclomagnesiation of 1,2-nonadecadiene 2 and tetrahydropyran ether 13,14-pentadecadienol 3 was carried out using EtMgBr in the presence of metallic Mg and a catalyst Cp2TiCl2 (10 mol%), through the stage of formation of magnesacyclopentane 4, the deuterolysis of which gives tetrahydropyran ether 14,17-Dideutero-(13Z,17Z)-tetraconte-13,17-dienol 5 in 84% yield. Successive reactions of removal of tetrahydropyranyl protection and oxidation of unsaturated deuterated alcohol 6 using Dess–Martin periodinan led to 14,17-Dideutero-(13Z,17Z)-tetrakont-13,17-dienal 7 in ~86% yield. As a result of the reaction of the previously synthesized lithium (trimethylsilyl)acetylenide with aldehyde 7 and removal of the trimethylsilyl protection with TBAF, racemic d2-lembehyne B is formed in ~92% yield. Oxidation of hydroxyl group 9 with Dess–Martin periodinane gives ketone 10, and subsequent stereoselective reduction leads to the target d2-lembehyne B 1 (Scheme 1).
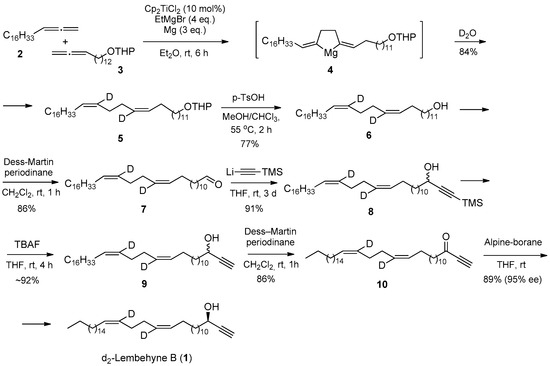
Scheme 1.
Complete synthesis d2-lembehyne B.
For the synthesized compound, the in vitro antitumor activity was assessed on Jurkat, K562, HL-60, and U937 cell lines and fibroblasts, including the determination of IC50 using flow cytometry and multiplex analysis.
3. Conclusions
Thus, we are the first to obtain a partially deuterated analogue of lembehyne B, using the reaction of Ti-catalyzed cross-cyclomagnesiation of 1,2-dienes (Dzhemilev reaction) at the key stage of synthesis, and also studied its antitumor activity using modern methods of flow cytometry and multiplex analysis.
4. Experimental Part
Commercially available reagents (Sigma-Aldrich «Sigma-Aldrich Corparation, PO Box 14508, Saint Louis, MO 63178 USA» and Acros Organics «Janssen-Pharmaceuticalaan 3a, 2440 Geel, Belgium») were used. Reactions with organomagnesium compounds were carried out under dried argon atmosphere. 1,2-dienes was prepared according to the known procedure. Reaction products were analyzed on a Carlo Erba chromatograph (a Hewlett Packard Ultra-1 glass capillary column, 25 m × 0.2 mm, flame ionization detector, operating temperature 50–170 °C, carrier gas helium). TLC was performed on Silufol UV-254 plates. Elemental composition of compounds was determined using a Carlo Erba-1106 instrument. Mass spectra were obtained using a Bruker MALDI-TOF/TOF Autoflex III instrument. Then, 1H and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer (100.62 MHz for 13C and 400.13 MHz for 1H). Chemical shifts of 1H and 13C nuclei (δ) are given relative to the residual signals of the deuterated solvent (δ 7.28 for protons and 77.2 for carbon nuclei).
Cross-cyclomagnesiation of nonadeca-1,2-diene (2) and 2-(pentadeca-13,14-dien-1-yloxy)tetrahydro -2H-pyran (3) with EtMgBr in the presence of Mg metal and Cp2TiCl2 catalyst. Diethyl ether (30 mL), nonadeca-1,2-diene (2) (1.27 g, 4.8 mmol), 2-(pentadeca-13,14-dien-1-yloxy) tetrahydro-2H-pyran (3) (1.23 g, 4.0 mmol), EtMgBr (16.0 mmol) (as 1.5 M solution in Et2O), Mg powder (0.29 g, 12.0 mmol), and Cp2TiCl2 (0.1 g, 0.4 mmol) were placed in a glass reactor with stirring under argon (~0 °C). The reaction mixture was warmed up to room temperature (20–22 °C) and stirred for 6 h. The reaction mixture was treated with D2O (20 mL) and extracted with diethyl ether (2 × 100 mL). The combined organic phases were dried over MgSO4 and filtered, and the solvents were removed under reduced pressure. Silica gel column chromatography (hexane/EtOAc (35/1)) of the residue gave compound 5 (1.98 g, 88 %) as a pale yellow oily liquid.
14,17-Dideutero-2-[(13Z,17Z)-tetratriaconta-13,17-dien-1-yloxy]tetrahydro-2H-pyran (5). Yeld 84%. Rf = 0.40. IR (film) υmax 724, 815, 1075, 1110, 1254, 1303, 1360, 1384, 1468, 2853, 2924 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.87 (3H, t, J = 6 Hz, CH3), 1.25–1.70 (48H, m, CH2), 1.78–1.85 (6H, m, CH2), 1.95–2.05 (8H, m, CH2), 3.32–3.87 (4H, m, CH2-O), 4.54–4.56 (1H, m, CH-O), 5.32–5.39 (2H, m, CH=). 13C NMR (100.62 MHz, CDCl3) δ: 14.07, 19.56, 22.68, 25.54, 26.27, 27.20, 27.28, 29.21, 29.25, 29.32, 29.38, 29.51, 29.56, 29.62, 29.72, 29.76, 30.73, 31.94, 61.99, 67.53, 98.61, 130.06. MS (MALDI-TOF), m/z: 577 [M]+. C39H72D2O2. Found (%): C, 81.26; H, 12.90. Calc. for C39H72D2O2 (%):C, 81.18; H, 13.27.
THP-deprotection of ether (5) was carried out with p-TsOH in CH2Cl2/MeOH using known method [17]. 14,17-Dideutero-(13Z,17Z)-tetratriaconta-13,17-dien-1-ol (6). Yeld 77%. Rf = 0.43 (hexane/EtOAc—5:1). IR (film) υmax 674, 729, 1034, 1078, 1124, 1159, 1180, 1204, 1354, 1382, 1441, 1662, 2853, 2924 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.91 (3H, t, J = 6 Hz, CH3), 1.26–1.37 (44H, m, CH2), 1.55–1.61 (2H, m, CH2), 1.98–2.10 (8H, m, CH2), 3.64–3.68 (2H, m, CH2-O), 5.40–5.42 (4H, m, CH=). 13C NMR (100.62 MHz, CDCl3) δ: 14.12, 22.70, 25.76, 27.24, 27.32, 29.28, 29.34, 29.41, 29.47, 29.55, 29.58, 29.62, 29.65, 29.71, 29.75, 31.94, 32.83, 63.09, 130.24. MS (MALDI-TOF), m/z: 492 [M]+. C34H64D2O. Found (%): C, 82.11; H, 13.44. Calc. for C34H64D2O (%): C, 82.85; H, 13.90.
The oxidation of the alcohol (6) with Dess-Martin periodinane was carried out according known procedure [18]. 14,17-Dideutero-(13Z,17Z)-tetratriaconta-13,17-dien-1-al (7). Yeld 86%. IR (film) υmax 721, 910, 1091, 1466, 1729, 2852, 2922, 3009 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.89 (3H, t, CH3, J = 6 Hz), 1.27–1.35 (44H, m, CH2), 1.60–1.67 (3H, m), 2.00–2.08 (8H, m, CH2), 2.40–2.44 (2H, m, CH2), 5.36–5.43 (4H, m, =CH), 9.76–9.77 (1H, t, O=CH, J = 6 Hz). 13C NMR (100.62 MHz, CDCl3) δ: 14.10, 22.09, 22.70, 27.23, 27.30, 29.29, 29.37, 29.44, 29.52, 29.56, 29.59, 29.62, 29.68, 29.72, 29.76, 31.94, 43.91, 130.16, 130.19, 202.24. MS (MALDI-TOF), m/z: 490 [M]+. C34H62D2O. Found (%): C, 83.44; H, 13.23. Calc. for C34H62D2O (%): C, 83.19; H, 13.55.
Procedure for preparation of alkyne (8). To a solution of 0.58 g (6 mmol) of trimethylsilyl acetylene, THF (10 mL) was added dropwise a solution of 4 ml n-BuLi (1.5 M in hexane) at −40 °C. The solution was stirred for 1 h at −40 to 0 °C. Then, the solution was added dropwise to THF solution of 1.5 g (3.08 mmol) dienal (7) at −10 °C. The reaction mixture was warmed up to room temperature (20–22 °C) and stirred for 3 days. The reaction mixture was treated with a 5% solution of NH4Cl in H2O (20 mL) and extracted with diethyl ether (2 × 100 mL). The combined organic phases were dried over MgSO4 and filtered, and the solvents were removed under reduced pressure. Silica gel column chromatography of the residue gave compound 8 (1.64 g, 91 %) as a pale yellow oily liquid.
16,19-Dideutero-(15Z,19Z)-1-(trimethylsilyl)hexatriaconta-15,19-dien-1-yn-3-ol (8). Yeld 91%. IR (film) υmax 550, 627, 655, 720, 781, 808, 890, 909, 965, 1022, 1306, 1377, 1464, 1654, 2116, 2835, 2924, 3008, 3313 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.18 (9H, т, CH3), 0.90 (3H, t, CH3, J = 6 Hz), 1.28–1.75 (49H, m, CH2), 1.91–2.14 (8H, m, CH2), 4.35 (1H, t, O-CH, J = 5 Hz), 5.36–5.43 (2H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ:−0.11, 14.13, 22.72, 25.14, 27.24, 27.32, 29.28–29.78, 31.97, 37.69, 62.76, 89.01, 107.19, 130.16. MS (MALDI-TOF), m/z: 590 [M]+. C39H74OSiD2. Found (%): C, 79.68; H, 12.85. Calc. for C39H74OSiD2 (%): C, 79.51; H, 13.00.
Procedure for preparation of 16,19-Dideutero-(15Z,19Z)-dimethylhexatriaconta-15,19-dien-1 -yn-3-ol (9). To a solution of 1.17 g (2 mmol) of alkyne (8), THF (10 mL) was added TBAF (1M in THF, 1.2 equv.) at 0 °C, and then the solution was stirred for 6 h at room temperature. Then, the solution was added dropwise to THF solution of 1.5 g (3.08 mmol) dienal (7) at −10 °C. The reaction mixture was treated with saturated aq. NaCl and extracted with diethyl ether (2 × 50 mL). The combined organic phases were dried over MgSO4 and filtered, and the solvents were removed under reduced pressure. Silica gel column chromatography of the residue gave compound 1 (1.07 g, 99 %) as a colorless powder.
16,19-Dideutero-(15Z,19Z)-dimethylhexatriaconta-15,19-dien-1-yn-3-ol (9). Yeld 92%.
+0.43 (c 0.3, CHCl3). IR (film) υmax 551, 627, 655, 721, 781, 809, 890, 909, 965, 1022, 1307, 1377, 1464, 1654, 2116, 2835, 2924, 3008, 3311 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.90 (3H, t, CH3, J = 6.7 Hz), 1.30–1.50 (44H, m, CH2), 1.66–1.79 (2H, m, CH2), 1.88–2.16 (8H, m, CH2), 2.48 (1H, d, CH), 4.39 (1H, td, J = 7.0, 2.0 Hz), 5.40 (2H, t, =CH, J = 6.7 Hz). 13C NMR (100.62 MHz, CDCl3) δ: 14.14, 22.71, 25.03, 27.25, 27.32, 29.22, 29.26, 29.35, 29.39, 29.54, 29.59, 29.65, 29.68, 29.77, 31.95, 37.68, 62.37, 72.82, 85.03, 130.24. MS (MALDI-TOF), m/z: 516 [M]+. C36D2H64O. Found (%): C, 84.30; H, 12.57. Calc. for C36D2H64O (%): C, 84.11; H, 12.78.
The oxidation of the alcohol (9) with Dess-Martin periodinane was carried out according [18]. (15Z,19Z)-16,19-Dideutero-(15Z,19Z)-hexaconta-15,19-dien-1-yl-3-one (10). Yield 86%. IR (film) υmax 553, 627, 655, 720, 786, 807, 890, 909, 965, 1022, 1306, 1377, 1461, 1654, 2116, 2835, 2924, 3008, 3312 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.90 (3H, t, CH3, J = 6 Hz), 1.23–1.48 (44H, m, CH2), 1.64–1.72 (2H, m, CH2), 2.04–2.10 (8H, m, CH2), 2.58–2.62 (2H, m, CH2), 3.22 (1H, s, CH), 5.39–5.42 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ: 187.5, 130.4, 130.3, 129.1, 81.5, 78.2, 45.5, 31.9, 29.3–29.7 (signals of 19C), 28.9, 27.4, 27.3, 22.7, 23.8, 14.1. MS (MALDI-TOF), m/z: 514 [M]+. C36D2H62O.Found (%): C, 84.14; H, 12.80. Calc. for C36D2H62O (%): C, 84.19; H, 12.82.
The stereoselective reduction of ketone 10 with B-3-pinanyl-9-borabicyclo[3.3.1]nonane (Alpine-borane reagent) was carried out accordingprocedure [19]. d2-Lembehyne B (1). Yield 89% (95% ee).
+0.43 (c 0.3, CHCl3). IR (film) υmax 550, 627, 655, 721, 781, 809, 890, 909, 965, 1022, 1307, 1377, 1464, 1654, 2116, 2835, 2924, 3008, 3310 cm−1. 1H NMR (400 MHz, CDCl3) δ: 0.90 (3H, t, CH3, J = 7 Hz), 1.23–1.54 (44H, m, CH2), 1.71–1.75 (2H, m, CH2), 2.04–2.10 (8H, m, CH2), 4.39 (1H, td, J = 7.0, 2.0 Hz), 5.39–5.41 (4H, m, =CH). 13C NMR (100.62 MHz, CDCl3) δ: 130.4, 129.2, 85.0, 72.8, 62.4, 37.7, 31.9, 29.3–29.7 (signals of 19C), 29.2, 27.4, 27.3, 25.0, 22.7, 14.1.
Author Contributions
Conceptualization, U.M.D. and L.U.D.; methodology, A.A.M.; validation, E.K.M.; resources, E.K.M.; data curation, U.M.D.; writing—original draft preparation, E.K.M. and A.A.M.; writing—review and editing, U.M.D. and L.U.D.; visualization, E.K.M.; supervision, U.M.D.; project administration, A.A.M.; funding acquisition, A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was done within approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0075.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The structural studies of the synthesized compounds were performed with the use of Collective Usage Centre “Agidel” at the Institute of Petrochemistry and Catalysis of RAS. The biological studies of bicycles were done in the Laboratory of Molecular Design and Drug Bioscreening at the Institute of Petrochemistry and Catalysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aoki, S.; Matsui, K.; Wei, H.; Murakami, N.; Kobayashi, M. Structure–Activity Relationship of Neuritogenic Spongean Acetylene Alcohols, Lembehynes. Tetrahedron 2002, 58, 5417–5422. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V. Acetylenic Aquatic Anticancer Agents and Related Compounds. Nat. Prod. Commun. 2006, 1, 405–429. [Google Scholar] [CrossRef] [Green Version]
- Dzhemileva, L.U.; D’yakonov, V.A.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Dzhemilev, U.M. The first total synthesis of the marine acetylenic alcohol, lembehyne B—A selective inducer of early apoptosis in leukemia cancer cells. Org. Biomol. Chem. 2017, 15, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Syroeshkin, A.V.; Elizarova, T.E.; Pleteneva, T.V.; Uspenskaya, E.V.; Levitskaya, O.V.; Zlatskiy, I.A.; Maksimova, T.V. The Influence of Deuterium on the Properties of Pharmaceutical Substances. Drag Dev. Regist. 2020, 9, 24–32. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Ibragimov, A.G.; Khalilov, L.M.; Dzhemilev, U.M. Novel Mg-organic reagents in organic sinthesis.Cp2TiCl2 catalized intermolecular cyclomagnesiation of cyclic and acyclic 1,2-dienes using Grignard reagents. Tetrahedron 2008, 64, 10188–10194. [Google Scholar] [CrossRef]
- Dyakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Khalilov, L.M.; Dzhemilev, U.M. Cyclomagnesiation of N-Containing 1,2-Dienes Using Grignard Reagents Catalyzed by Cp2TiCl2. Russ. J. Org. Chem. 2012, 48, 357–361. [Google Scholar]
- Dyakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Khalilov, L.M.; Dzhemilev, U.M. Synthesis and transformation of metalcycles. Communication 41. Cyclomagnesiation of O-containing 1,2-dienes with Grignard reagents in the presence of Cp2TiCl2. Russ. Chem. Bull. Int. Ed. 2012, 10, 1928–1934. [Google Scholar]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Dzhemilev, U.M. Novel organomagnesium reagents in synthesis. Catalytic cyclomagnesiation of allenes in the synthesis of N-, O-, and Si-substituted 1Z,5Z-dienes. Tetrahedron 2013, 69, 8516–8526. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.Kh.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’yakonov, V.A.; Makarov, A.A.; Mulukova, A.R.; Dzhemilev, U.M. Catalytic cross cyclomagnesiation of 1,2-dienes in the synthesis of Z,Z-dienoic alcohols and 5Z,9Z-dienoic acids. Russ. Chem. Bull. Int. Ed. 2015, 9, 2135–2140. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Makarov, A.A.; Dzhemilev, U.M. Ti-Catalyzed cross-cyclomagnesiation of 1,2-dienes in the stereoselective synthesis of insect pheromones. Tetrahedron Lett. 2017, 58, 1755–1757. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. The first total synthesis of Lembehyne B. Mendeleev Commun. 2017, 27, 122–124. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. Total Synthesis of Neuritogenic Alkynes: Lembehyne B and Key Intermediate of Lembehyne A. Chem. Sel. 2017, 2, 1211–1213. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Makarova, E.K.; D’yakonov, V.A.; Dzhemilev, U.M. New 1,3-Diynoic Derivatives of Natural Lembehyne B: Stereoselective Synthesis, Anticancer and Neuritogenic Activity. ACS Omega 2020, 5, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Andreev, E.N.; Makarova, E.K.; Dzhemilev, U.M. Total Synthesis of Natural Lembehyne C and Investigation of Its Cytotoxic Properties, J. Nat. Prod. 2020, 83, 2399–2409. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Tuktarova, R.A.; Dzhemilev, U.M. Ti-Catalyzed Cross-Cyclomagnesiation of 1,2-Dienes in the Total Z,Z,Z-Stereoselective Synthesis of Natural Acetogenin—Chatenaytrienin-1. ACS Omega 2019, 4, 14085–14091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuisle, O.; Quiñoá, E.; Riguera, R. A general methodology for automated solid-phase synthesis of depsides and depsipeptides. Preparation of a valinomycin analogue. J. Org. Chem. 1999, 64, 8063–8075. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.D.; Schreiber, S.L. Acceleration of the Dess-Martin Oxidation by Water. J. Org. Chem. 1994, 59, 7549–7552. [Google Scholar] [CrossRef]
- Midland, M.M.; Tramontano, A.; Kazubski, A.; Graham, R.S.; Tsai, D.J.S.; Cardin, D.B. Asymmetric reductions of propargyl ketones. An effective approach to the synthesis of optically active compounds. Tetrahedron 1984, 40, 1371–1380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).