Development and Evaluation of Eberconazole-Loaded Niosomes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preformulation Studies
2.2.2. Preparation of Blank and Drug-Loaded Niosomal Vesicles
2.3. Characterization Methods
3. Results and Discussion
3.1. Preformulation Studies
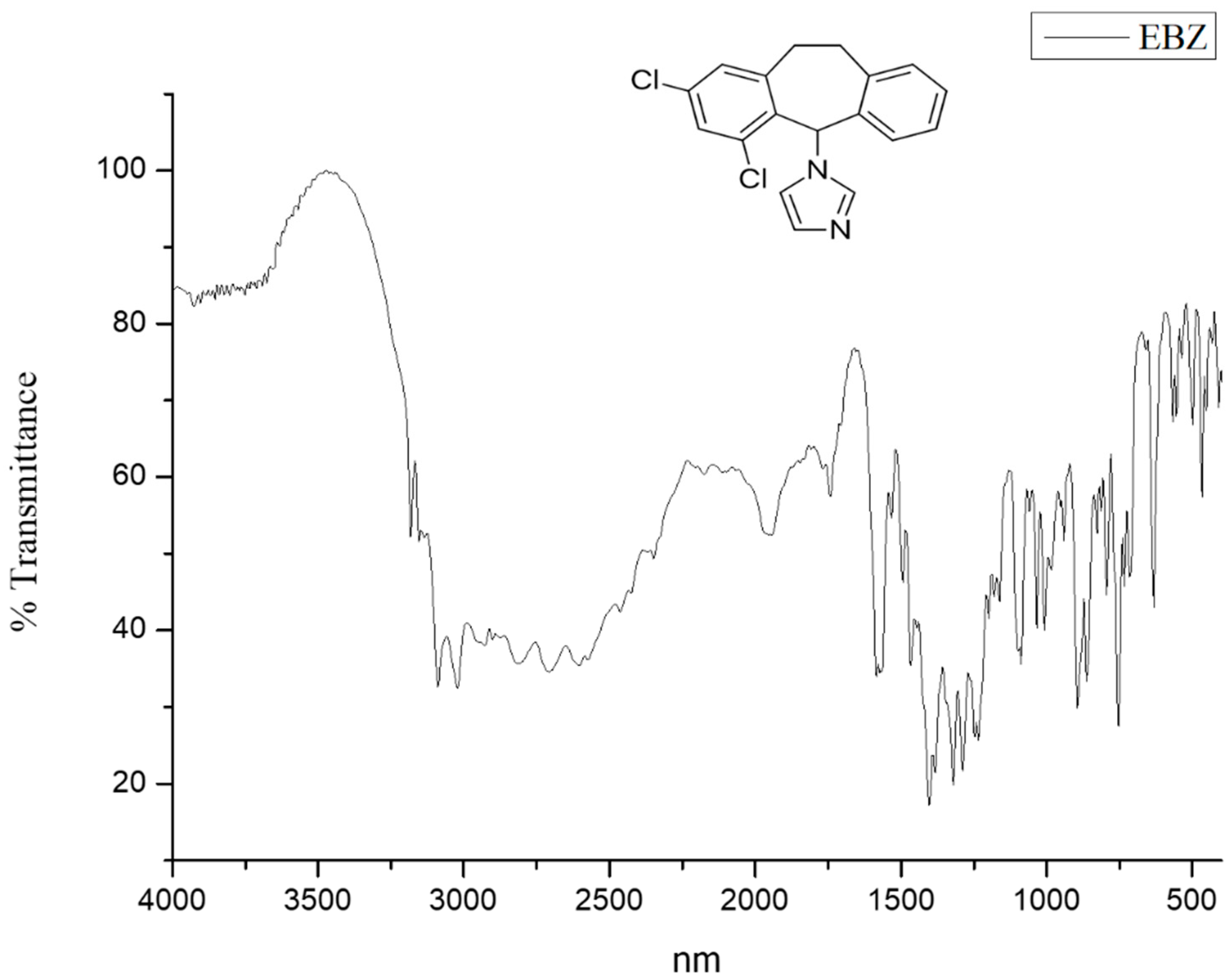
3.2. FTIR Study
3.3. Standard Plot
3.4. Morphological Evaluation
3.5. Particle Size and Zeta Potential
3.6. Entrapment Efficiency
3.7. FTIR Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagh, V.D.; Deshmukh, O.J. Itraconazole niosomes drug delivery system and its antimycotic activity against Candida albicans. Int. Sch. Res. Not. 2012, 2012, 653465. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2011, 25, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Rapp, R.P. Changing Strategies for the Management of Invasive Fungal Infections. Pharmacotherapy 2004, 24, 4–28. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkader, H.; Ismail, S.; Kamal, A.; Alany, R.G. Design and Evaluation of Controlled-Release Niosomes and Discomes for Naltrexone Hydrochloride Ocular Delivery. J. Pharm. Sci. 2011, 100, 1833–1846. [Google Scholar] [CrossRef]
- Liang, X.; Mao, G.; Ng, K.Y.S. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J. Colloid Interface Sci. 2004, 278, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Caminiti, R.; Marianecci, C.; Carafa, M.; Santucci, E.; de Sanctis, S.C.; Caracciolo, G. Effect of Cholesterol on the Formation and Hydration Behavior of Solid-Supported Niosomal Membranes. Langmuir 2010, 26, 2268–2273. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.A.E.A.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro–in vivo evaluation. Pharm. Dev. Technol. 2019, 24, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, J.M.; Mendez, R.; López-Jodra, O.; Morera, Y.; Espasa, M.; Jimenez, T.; Lagunas, C. In vitro susceptibilities of clinical yeast isolates to the new antifungal eberconazole compared with their susceptibilities to clotrimazole and ketoconazole. Antimicrob. Agents Chemother. 1999, 43, 1258–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, T.R.; López, S.; Rodríguez, C.; del Rio, R.; Badell, A.; Gratacós, M.R. Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: Multicenter, randomized, double-blind, comparative trial with miconazole 2% cream. Int. J. Dermatol. 2006, 45, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Porfire, A.; Muntean, D.M.; Rus, L.; Sylvester, B.; Tomuţă, I. A quality by design approach for the development of lyophilized liposomes with simvastatin. Saudi Pharm. J. 2017, 25, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and characterization of niosomes containing ribavirin for liver targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.V.K.; Reddy, P.R.; Lee, Y.I.; Kim, C. Synthesis and characterization of chitosan-PEG-Ag nanocomposites for antimicrobial application. Carbohydr. Polym. 2012, 87, 920–925. [Google Scholar]
- Asha, K.; Vikash, D. Formulation and evaluation of zidovudine loaded chitosan microspheres for controlled release. Int. J. Drug Dev. Res. 2012, 4, 96–105. [Google Scholar]
- Shirsand, S.; Para, M.; Nagendrakumar, D.; Kanani, K.; Keerthy, D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int. J. Pharm. Investig. 2013, 2, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodahadu-Bangera, L.S.; Martis, J.; Mittal, R.; Krishnankutty, B.; Kumar, N.; Bellary, S.; Varughese, S.; Rao, P.K. Eberconazole-Pharmacological and clinical review. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Fàbrega, C.; Grijalvo, S.; Vitiello, G.; D’Errico, G.; Eritja, R.; Montesarchio, D. AS1411-decorated niosomes as effective nanocarriers for Ru(III)-based drugs in anticancer strategies. J. Mater. Chem. B 2018, 6, 5368–5384. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparajay, P.; Dev, A. Development and Evaluation of Eberconazole-Loaded Niosomes. Chem. Proc. 2022, 8, 28. https://doi.org/10.3390/ecsoc-25-11664
Aparajay P, Dev A. Development and Evaluation of Eberconazole-Loaded Niosomes. Chemistry Proceedings. 2022; 8(1):28. https://doi.org/10.3390/ecsoc-25-11664
Chicago/Turabian StyleAparajay, Priyadarshi, and Abhimanyu Dev. 2022. "Development and Evaluation of Eberconazole-Loaded Niosomes" Chemistry Proceedings 8, no. 1: 28. https://doi.org/10.3390/ecsoc-25-11664
APA StyleAparajay, P., & Dev, A. (2022). Development and Evaluation of Eberconazole-Loaded Niosomes. Chemistry Proceedings, 8(1), 28. https://doi.org/10.3390/ecsoc-25-11664





