Synthesis of a Symmetrical tris-Tetrazole as Isostere of a Tricarboxylic Acid: Behind New Tridentate Ligands for MOFs †
Abstract
:1. Introduction
2. Results and Discussion
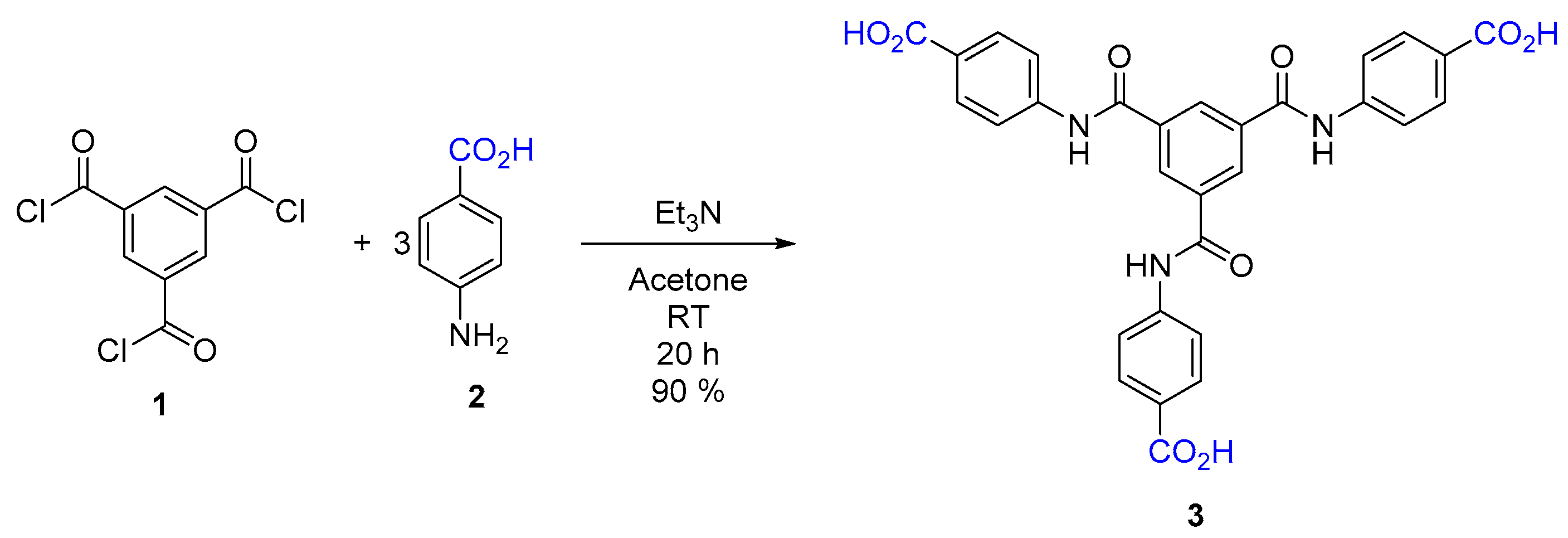
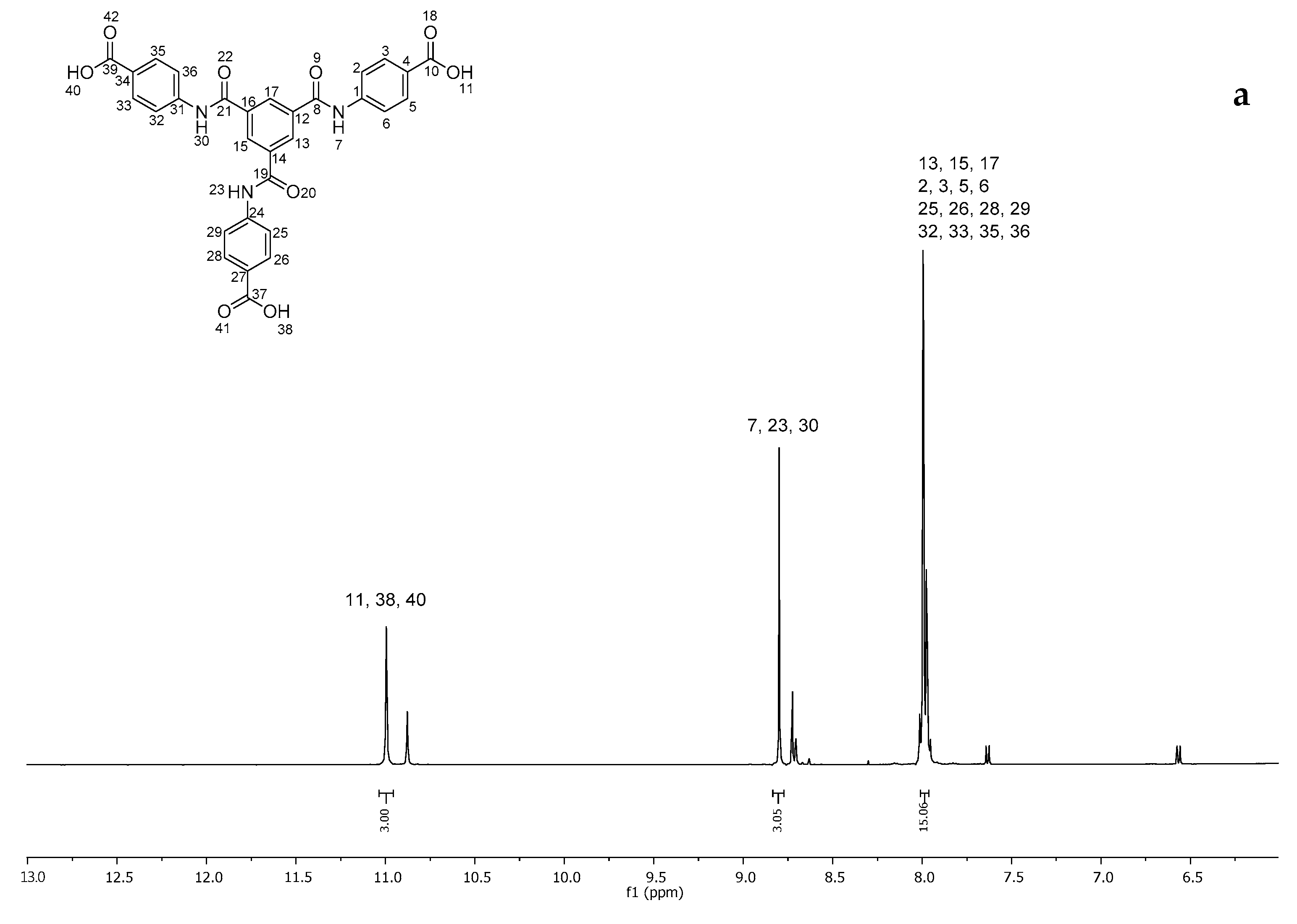
2.1. Synthesis of 4,4′,4″-((Benzene-1,3,5-tricarbonyl)tris(azanediyl))tribenzoic Acid (3)
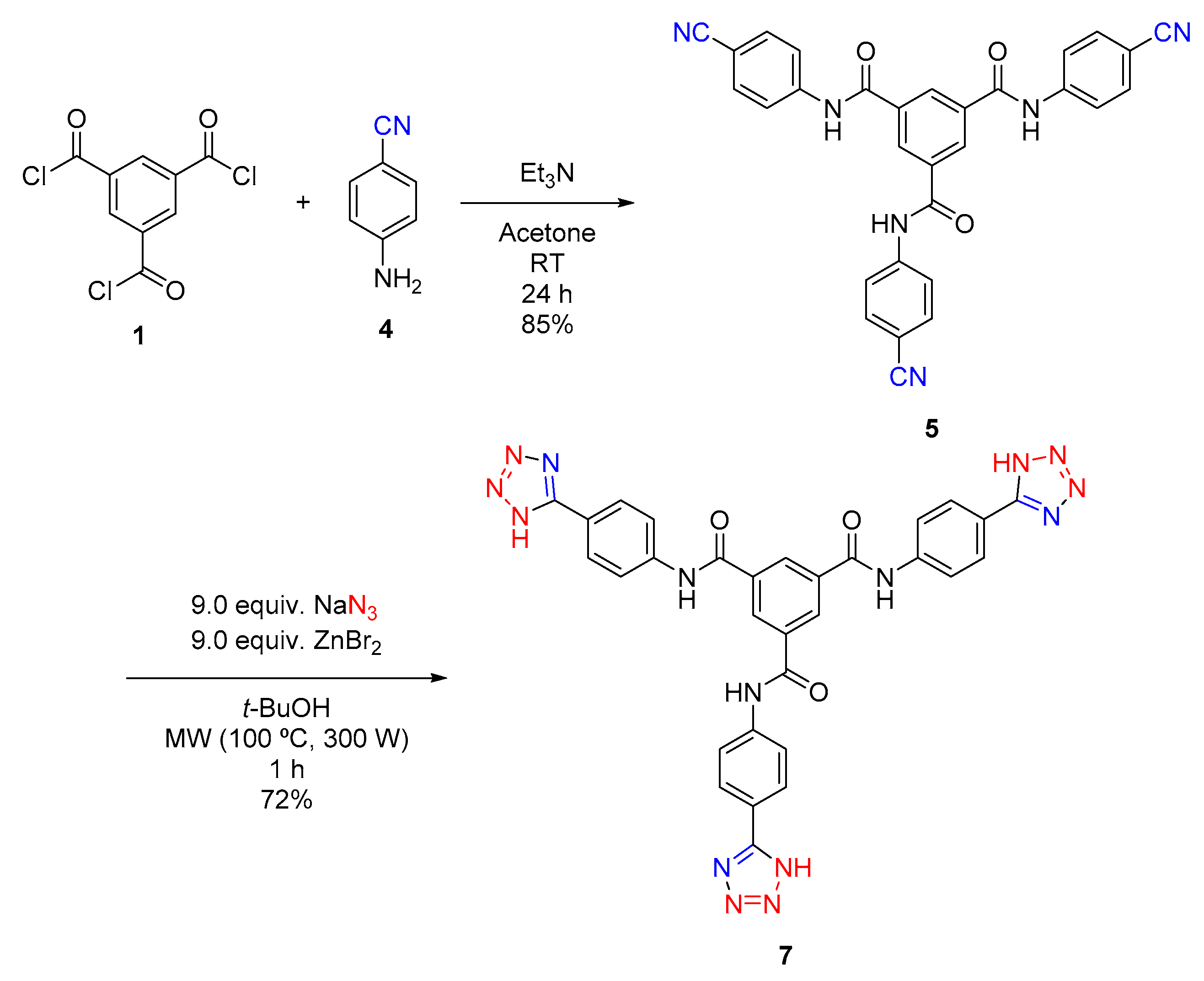
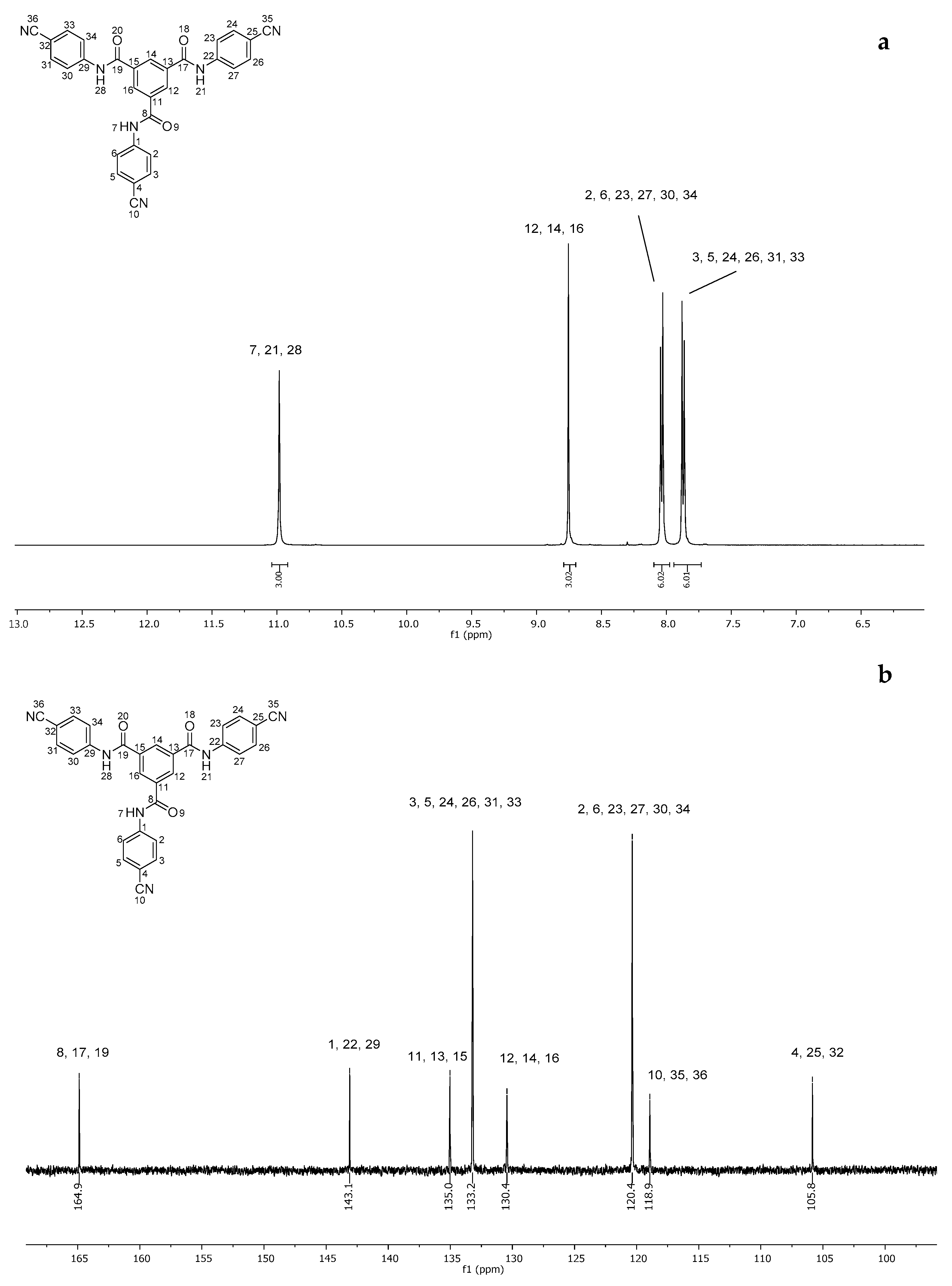
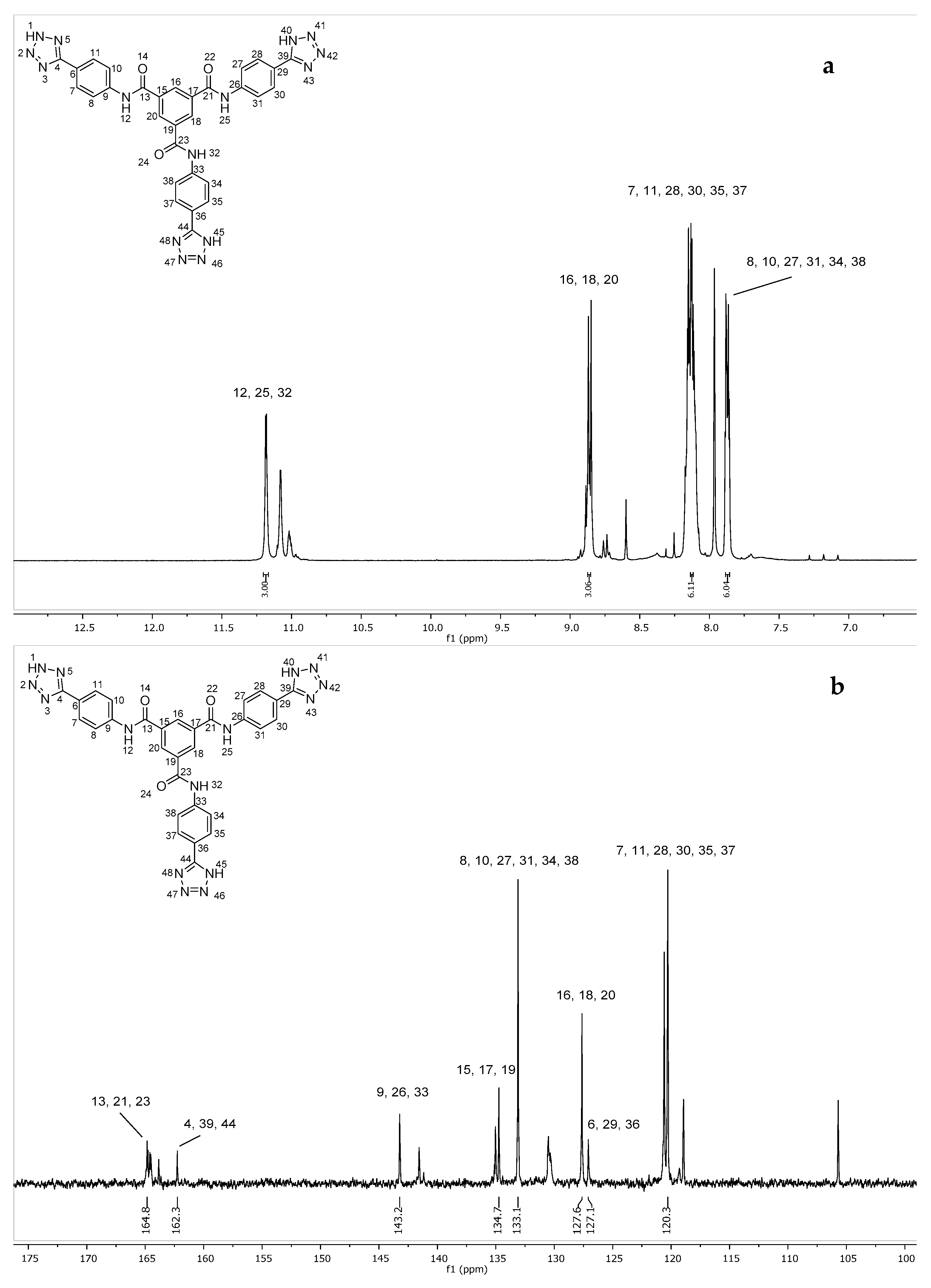
2.2. Synthesis of N1,N3-Bis(4-(1H-tetrazol-5-yl)phenyl)-N5-(4-(2H-tetrazol-5-yl)phenyl)benzene-1,3,5-tricarboxamide (7)
3. Conclusions
4. Experimental Section
4.1. General Information, Instrumentation and Chemicals
4.2. Synthesis of 4,4′,4″-((Benzene-1,3,5-tricarbonyl)tris(azanediyl))tribenzoic Acid (3)
4.3. Synthesis of N1,N3,N5-Tris(4-cyanophenyl)benzene-1,3,5-tricarboxamide (5)
4.4. Synthesis of N1,N3-Bis(4-(1H-tetrazol-5-yl)phenyl)-N5-(4-(2H-tetrazol-5-yl)phenyl)benzene-1,3,5-tricarboxamide (7)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Wei, Z.; Gu, Z.G.; Liu, T.L.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Marquéz, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Beuerle, F.; Feldman, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2015, 216, 171–199. [Google Scholar] [CrossRef]
- López-Olvera, A.; Flores, J.G.; Aguilar-Pliego, J.; Brozek, C.K.; Gutiérrez-Alejandre, A.; Ibarra, I.A. Chemical Transformation of H2S within the Pores of Metal–Organic Frameworks: Formation of Polysulfides. Chem. Mater. 2021, 33, 6269–6276. [Google Scholar] [CrossRef]
- Martínez-Ahumada, E.; He, D.; Berryman, V.; López-Olvera, A.; Hernandez, M.; Jancik, V.; Martis, V.; Vera, M.A.; Lima, E.; Parker, D.J.; et al. SO2 Capture Using Porous Organic Cages. Angew. Chem. Int. Ed. 2021, 60, 17556–17563. [Google Scholar] [CrossRef] [PubMed]
- Cotlame-Salinas, V.; López-Olvera, A.; Islas-Jácome, A.; González-Zamora, E.; Ibarra, I.A. CO2 capture enhancement in MOFs via the confinement of molecules. React. Chem. Eng. 2021, 6, 441–453. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Jie-Peng, Z.; Yue-Biao, Z.; Jian-Bin, L.; Xiao-Ming, C. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar]
- Ballatore, C.; Huryn, D.; Smith III, A. Carboxylic acid (bio)isosteres in drug design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dojer, B.; Pevec, A.; Belaj, F.; Kristl, M. Two New Zinc(II) Acetates with 3- and 4-Aminopyridine: Syntheses and Structural Properties. Acta Chim. Slov. 2015, 62, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design Analysis and Application, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2009; pp. 172–178. [Google Scholar]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gámez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Lennartson, A.; McKenzie, C.J. Bridging nitrile groups in a metal–organic framework. J. Coord. Chem. 2012, 65, 4194–4202. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-Substituted 1H-Tetrazoles from Nitriles in Water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Park, M.; Hong, S.; Lah, M.S. A designed metal–organic framework based on a metal–organic polyhedron. Chem. Commun. 2008, 20, 2340–2342. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, G.; Zheng, B.; Wang, Z.; Bai, J. A porous amide-functionalized pto-type MOF exhibiting selective capture and separation of cationic MB dye. J. Coord. Chem. 2021, 74, 241–251. [Google Scholar] [CrossRef]
- Song, X.; Zou, Y.; Liu, X.; Oh, M.; Lah, M.S. A two-fold interpenetrated (3,6)-connected metal–organic framework with rutile topology showing a large solvent cavity. New J. Chem. 2010, 34, 2396–2399. [Google Scholar] [CrossRef] [Green Version]
- Howe, R.; Smalley, A.; Guttenplan, A.; Doggett, M.; Eddleston, M.; Tan, J.; Lloyd, G. A family of simple benzene 1,3,5-tricarboxamide (BTA) aromatic carboxylic acid hydrogels. Chem. Commun. 2013, 49, 4268–4270. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Higuchi, M.; Foo, M.; Horike, S.; Rao, K.; Kitagawa, S. A Family of Rare Earth Porous Coordination Polymers with Different Flexibility for CO2/C2H4 and CO2/C2H6 Separation. Inorg. Chem. 2013, 52, 8244–8249. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Reyes, J.C.; Islas-Jácome, P.; Gutiérrez-Carrillo, A.; Rincón-Guevara, M.A.; Suárez-Moreno, G.V.; Vázquez-Vera, Ó.; Lomas-Romero, L.; González-Zamora, E.; Islas-Jácome, A. Synthesis of a Symmetrical tris-Tetrazole as Isostere of a Tricarboxylic Acid: Behind New Tridentate Ligands for MOFs. Chem. Proc. 2022, 8, 25. https://doi.org/10.3390/ecsoc-25-11751
Flores-Reyes JC, Islas-Jácome P, Gutiérrez-Carrillo A, Rincón-Guevara MA, Suárez-Moreno GV, Vázquez-Vera Ó, Lomas-Romero L, González-Zamora E, Islas-Jácome A. Synthesis of a Symmetrical tris-Tetrazole as Isostere of a Tricarboxylic Acid: Behind New Tridentate Ligands for MOFs. Chemistry Proceedings. 2022; 8(1):25. https://doi.org/10.3390/ecsoc-25-11751
Chicago/Turabian StyleFlores-Reyes, Julio C., Perla Islas-Jácome, Atilano Gutiérrez-Carrillo, Mónica A. Rincón-Guevara, Galdina V. Suárez-Moreno, Óscar Vázquez-Vera, Leticia Lomas-Romero, Eduardo González-Zamora, and Alejandro Islas-Jácome. 2022. "Synthesis of a Symmetrical tris-Tetrazole as Isostere of a Tricarboxylic Acid: Behind New Tridentate Ligands for MOFs" Chemistry Proceedings 8, no. 1: 25. https://doi.org/10.3390/ecsoc-25-11751
APA StyleFlores-Reyes, J. C., Islas-Jácome, P., Gutiérrez-Carrillo, A., Rincón-Guevara, M. A., Suárez-Moreno, G. V., Vázquez-Vera, Ó., Lomas-Romero, L., González-Zamora, E., & Islas-Jácome, A. (2022). Synthesis of a Symmetrical tris-Tetrazole as Isostere of a Tricarboxylic Acid: Behind New Tridentate Ligands for MOFs. Chemistry Proceedings, 8(1), 25. https://doi.org/10.3390/ecsoc-25-11751





