Study on the Effect of the Ligand Structure in Palladium Organometallic Catalysts in the Suzuki–Miyaura Cross-Coupling Reaction †
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979, 19, 866–867. [Google Scholar] [CrossRef]
- Kostas, I.D.; Steele, B.R. Thiosemicarbazone Complexes of Transition Metals as Catalysts for Cross-Coupling Reactions. Catalysts 2020, 10, 1107. [Google Scholar] [CrossRef]
- Jose, D.E.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent studies in Suzuki-Miyaura cross-coupling reactions with the aid of phase transfer catalysts. J. Organomet. Chem. 2020, 927, 121538. [Google Scholar] [CrossRef]
- Kadu, B.S. Suzuki–Miyaura cross coupling reaction: Recent advancements in catalysis and organic synthesis. Catal. Sci. Technol. 2021, 11, 1186–1221. [Google Scholar] [CrossRef]
- Munín, P.; Fernández-Figueiras, A.; Lucio, F.; Reigosa, F.; Vila, J.M.; Ortigueira, J.M.; Pereira, M.T. Preparation and characterization of thiosemicarbazone ligands and study of their iron and palladium derivatives. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Online, 1 November 2017; MDPI: Basel, Switzerland. [Google Scholar] [CrossRef][Green Version]
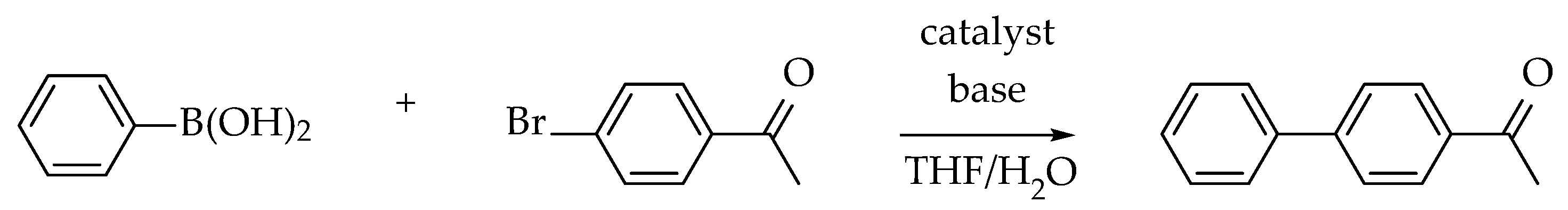
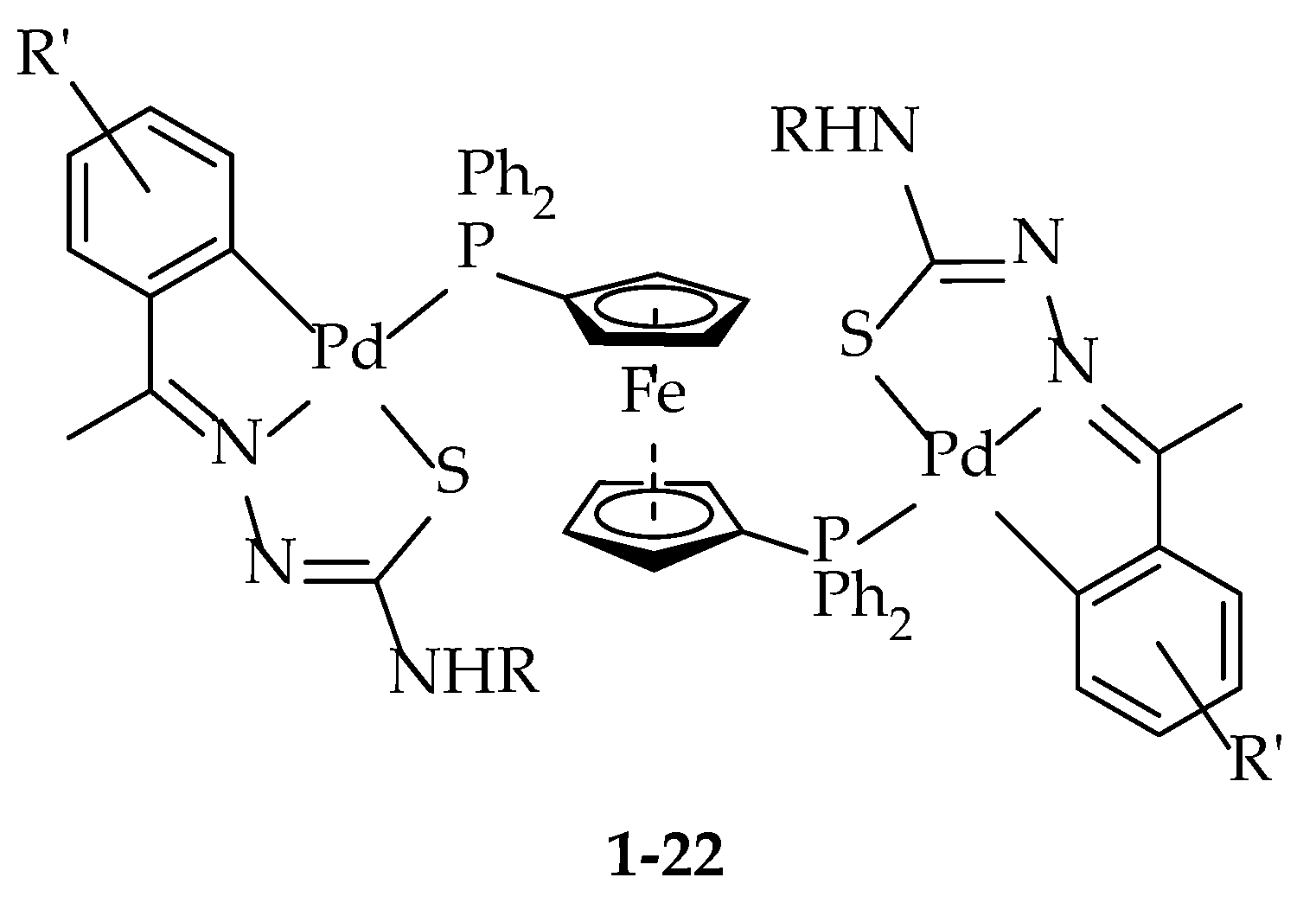
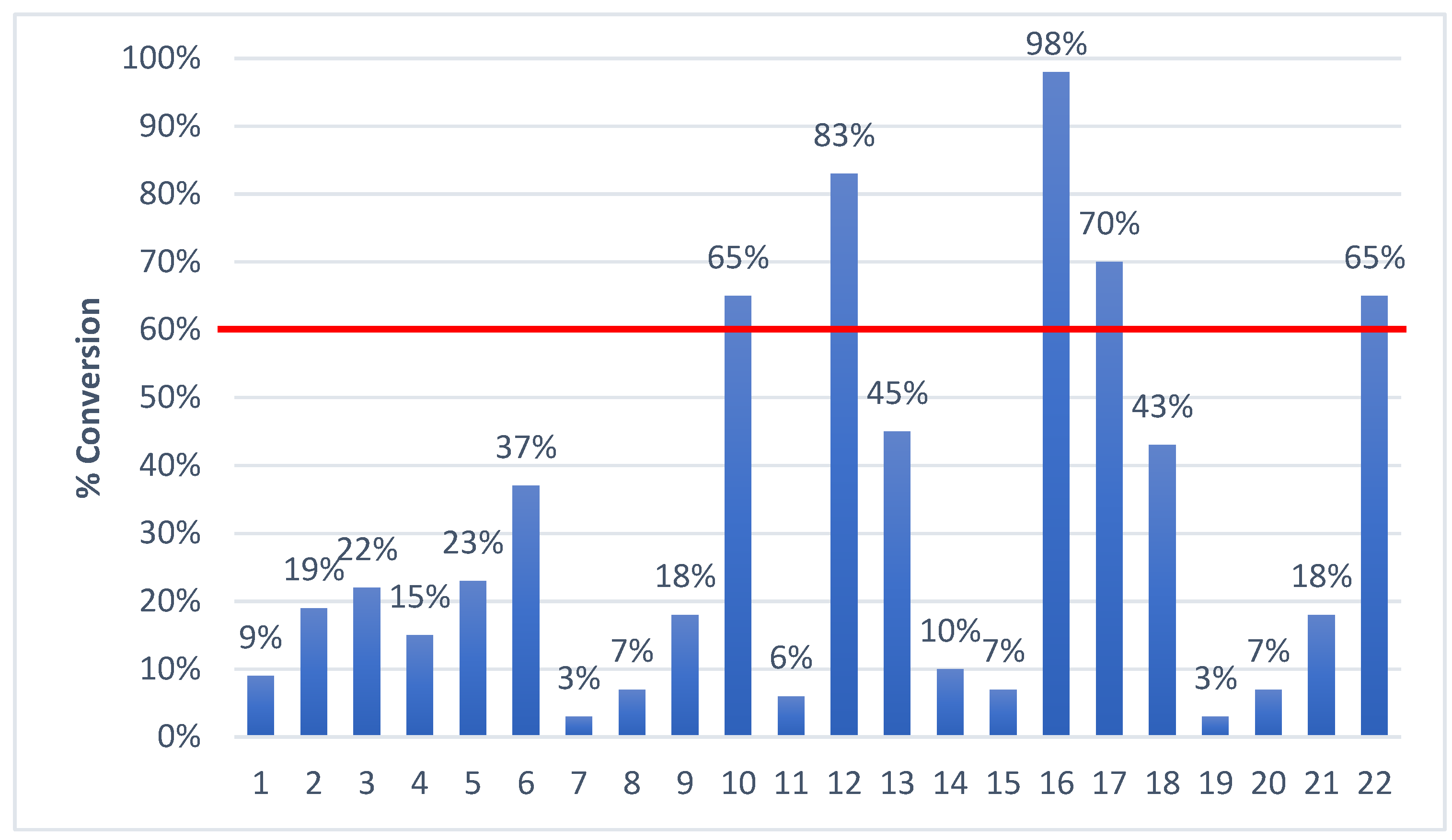
| Catalyst Number | Substituent R’ | 1 Substituent R |
|---|---|---|
| 1 | 4-Br | H |
| 2 | 4-Br | Me |
| 3 | 4-Br | Et |
| 4 | 4-OMe | H |
| 5 | 4-OMe | Me |
| 6 | 4-OMe | Et |
| 7 | 3-OMe | H |
| 8 | 3-OMe | Me |
| 9 | 3-OMe | Et |
| 10 | 3-OMe | Ph |
| 11 | 3,4-OMe | H |
| 12 | 3,4-OMe | Me |
| 13 | 3,4-OMe | Et |
| 14 | 3,4-OMe | Ph |
| 15 | 2,4-OMe | H |
| 16 | 2,4-OMe | Me |
| 17 | 2,4-OMe | Et |
| 18 | 2,4-OMe | Ph |
| 19 | 2,3,4-OMe | H |
| 20 | 2,3,4-OMe | Me |
| 21 | 2,3,4-OMe | Et |
| 22 | 2,3,4-OMe | Ph |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munín-Cruz, P.; Rúa-Sueiro, M.; Ortigueira, J.M.; Pereira, M.T.; Vila, J.M. Study on the Effect of the Ligand Structure in Palladium Organometallic Catalysts in the Suzuki–Miyaura Cross-Coupling Reaction. Chem. Proc. 2022, 8, 19. https://doi.org/10.3390/ecsoc-25-11730
Munín-Cruz P, Rúa-Sueiro M, Ortigueira JM, Pereira MT, Vila JM. Study on the Effect of the Ligand Structure in Palladium Organometallic Catalysts in the Suzuki–Miyaura Cross-Coupling Reaction. Chemistry Proceedings. 2022; 8(1):19. https://doi.org/10.3390/ecsoc-25-11730
Chicago/Turabian StyleMunín-Cruz, Paula, Marcos Rúa-Sueiro, Juan Manuel Ortigueira, María Teresa Pereira, and José Manuel Vila. 2022. "Study on the Effect of the Ligand Structure in Palladium Organometallic Catalysts in the Suzuki–Miyaura Cross-Coupling Reaction" Chemistry Proceedings 8, no. 1: 19. https://doi.org/10.3390/ecsoc-25-11730
APA StyleMunín-Cruz, P., Rúa-Sueiro, M., Ortigueira, J. M., Pereira, M. T., & Vila, J. M. (2022). Study on the Effect of the Ligand Structure in Palladium Organometallic Catalysts in the Suzuki–Miyaura Cross-Coupling Reaction. Chemistry Proceedings, 8(1), 19. https://doi.org/10.3390/ecsoc-25-11730






