Abstract
In the present work, the synthesis and antimicrobial activity of new thiazole substituted 1,3,4-oxadiazole derivatives was achieved. The reaction of different thioamides with ethyl 4-chloro-3-oxobutanoate (4-chloro ethyl acetoacetate) provided ethyl 2-(2-arylthiazol-4yl)acetate, which on subsequent reaction with hydrazine hydrate in absolute ethanol afforded 2-(2-arylthiazol-4-yl)acetohydrazide. 2-(2-arylthiazol-4-yl)acetohydrazide, on reaction with CS2 and KOH in aqueous ethanol, cyclized to form 5-((2-arylthiazol-4-yl)methyl)-1,3,4-oxadiazole-2-thiol. Finally, 5-((2-arylthiazol-4-yl)methyl)-1,3,4-oxadiazole-2-thiol was further treated with α-halo ketones at room temperature to achieve the target compounds. Most of the compounds showed good antibacterial activity, as well as antifungal activity.
1. Introduction
It is observed from the relevant literature that thiazole heterocycle is an important moiety accompanied by numerous remarkable biological activities t with thiazole derivatives. Large uses of thiazole originated during the development of drugs for the treatment of allergies [1], inflammation [2], HIV infections [3], and, more recently, for the treatment of pain [4]. Thiazole has also been used as a new inhibitor of bacterial DNA gyrase B [5], as well as for the following purposes: antitumor [6], antibiotic [7,8,9,10], anti-inflammatory [11], antibacterial [12], antifungal [13], antitubercular [14,15,16], and antiviral [17]. It has also been used as a peroxisome proliferator-activated receptor (PPAR) α/γ/δ pan agonist [18].
Furthermore, thiazole heterocycles are a noteworthy class of heterocyclic compounds that are present in several important biologically dynamic drug molecules, such as Ritonavir as an antiretroviral drug, Sulfathiazole as an antimicrobial drug, Tiazofurin as an antineoplastic drug, and Abafungin as an antifungal drug [19]. Thiazole-containing heterocycles perform various biological activities, such as antihypertensive, antimicrobial, antifungal, anti-HIV, anticonvulsant, and anti-inflammatory activities [20,21,22,23,24]. Derivatives of thiazole are also well-known to carry out anticancer activities [25,26,27]. Thiazole derivatives also perform anti-inflammatory [28,29], antibacterial [30], antihypertensive [31], antituberculosis [32], analgesic [33], and anticonvulsant activities [34].
A literature search revealed that an oxadiazole heterocycle clubbed with thiazole showed different biological activities, such as antimicrobial, antitumor, and antifungal activities [35,36,37,38], stearyl-CoA desaturase inhibition activity [39], antimicrobial and antitubercular activity [36,40], anti-proliferative, anti-mitotic, and microtubule destabilizing activities [41], and anti-micobacterial activity [42]. These results encouraged us to consider new thiazole-containing 1,3,4-oxadiazole derivatives and to monitor them for antibacterial and antifungal activities. In the present work, we report the synthesis and antimicrobial activity of new thiazole-substituted 1,3,4-oxadiazole derivatives.
2. Results and Discussion
The structure of ethyl-2(2-arylthiazol-4yl) acetate 2a–b was confirmed by the appearance of a band at 1725–30 cm−1, due to C=O stretching of the ester functional group. The structures of compounds 3a–b were confirmed by absorption bands in the regions of 3180–3320 cm−1 and 1680–1690 cm−1, due to C=O and NHNH2. The cyclization reaction of compounds 3a–b with CS2 in the presence of KOH to form 1,3,4-oxadiazoles 4a–b was confirmed by the disappearance of bands at 3180–3320 cm−1 and 1680–1690 cm−1 and by the appearance of a new band at 2450–2510 cm−1, due to SH stretching. The structures of 4a–b were also confirmed by 1H NMR spectra that showed a broad singlet at 11 ppm, due to SH, a singlet at 7.1–7.2 ppm, due to a thiazolyl proton, a singlet at 4.4–4.5 ppm, due to CH2, and a multiplet at 7.4–8.2 ppm, due to aromatic protons. The conversion of 4a–b to the target compounds 5a–h (Scheme 1) was also confirmed by elemental analysis, IR, 1H NMR, 13C NMR, and MS. The IR spectra of these compounds showed bands at 1690–1700 cm−1, due to C=O. The 1H NMR spectra of compounds 5a–h showed two singlets at 4.4–4.5 ppm and 4.8–4.9 ppm, due to two CH2 groups, and one singlet in the region of 7.1–7.2 ppm, due to a thiazolyl proton, while the aromatic protons appeared as a multiplet at 7.4–8.2 ppm. The molecular ion peaks of all the title compounds were obtained from EI-MS. The presence of M+2 peaks were characteristic for the compounds, with chlorine and bromine atoms.
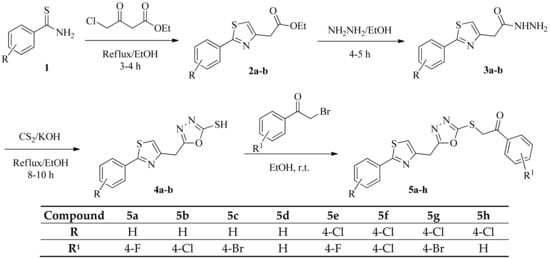
Scheme 1.
Synthetic route for synthesis of 5a–h.
3. Biological Results and Discussion
All of the synthesized compounds were screened for their antibacterial and antifungal activities. Most of the compounds 5a–h showed good antibacterial and antifungal activities, as shown in Table 1. The antimicrobial activity results clearly indicated that S-substituted thiazolyl-1,3,4-oxadiazole derivatives 5a–h showed enhanced antimicrobial activity, as compared to thiazolyl oxadiazole compound 4a and 4b in which the SH group is free. It was further observed that in compounds 5a and 5e, in which R1 is 4-F substituted, showed good antibacterial activity and antifungal activity, irrespective of the R group.

Table 1.
Antimicrobial screening of synthesized compounds.
4. Experimental
General procedure for synthesis of (3a–b): synthesized as per reference No. 38.
General procedure for the synthesis of 5-((2-arylthiazol-4-yl)methyl)-1,3,4-oxadiazole-2-thiol (4a–b) (Table 2).

Table 2.
Physical data of compounds 4a–b.
To a mixture of compound 3 (1 mmol) in ethanol (25 mL), carbon disulphide (1.3 mmol) and potassium hydroxide (1 mmol) were added. The reaction mixture was refluxed gently in water bath till evolution of H2S ceased. The progress of the reaction was monitored by TLC (30% Ethyl acetate/hexanes). After completion of the reaction, the solvent was completely removed and the residue was poured into water and acidified with concentrated HCl to obtain a solid product that was filtered, dried, and recrystallized from ethanol.
General procedure for the synthesis of 2-(5-(2-arylthiazol-4-yl)methyl)2-thiosubstituted-1,3,4-oxadiazole derivatives (5a–h).
To a stirred solution of compound 4a–b (1 mmol) in ethanol, substituted α-haloketones (1 mmol) were added. The reaction mixture was stirred at room temperature. The reaction progress was monitored by TLC (30% Ethyl acetate/hexanes). After completion, the reaction mixture was poured into crushed ice to obtain a solid product that was filtered, dried, and purified by column chromatography on silica gel using 2% of ethyl acetate/hexanes.
5. Spectral Data
2-(5-((2-Phenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-fluorophenyl) ethanone (5a) Yield: (68%); m.p.: 99–103 °C; IR (KBr, cm−1): 3120, 2931, 2854, 1690, 933 785, 688; 1H NMR (CDCl3, 300 MHz): δ 7.4–7.6 (m, 5H, Ar-H), 7.1 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 8.1 (d, J = 8.2 Hz, 2H, Ar-H), 7.2 (dd, J = 11.5 and 8.2 Hz, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 128.8, 129.0, 127.0, 132.0, 169.0, 109.0, 150.0, 32.0, 165.0, 169.0, 35.0, 183.0, 132.0, 130.0, [115.5, 115.8 (d, J = 22.5 Hz, 2C)], [165.3, 162.1 (d, J = 243 Hz, 1C)]; anal. calcd. for C20H14FN3O2S2: C, 58.38; H, 3.43; N, 10.21; found: C, 58.24; H, 3.28; N, 10.42; MS (EI, 70 eV): m/z (%) 411 (M+), 412 (M+1).
2-(5-((2-Phenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-chlorophenyl) ethanone (5b) Yield: (65%); m.p.: 105–108 °C; IR (KBr, cm−1): 3118, 2930, 1690, 938, 688; 1H NMR (CDCl3, 300 MHz): δ 7.4–7.6 (m, 5H, Ar-H), 7.1 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 7.7 (d, J = 8.4 Hz, 2H, Ar-H), 7.9 (d, J = 8.4, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 128.8, 129.0 (2C), 127.0 (2C), 132.0, 169.0, 109.0, 150.0, 32.0, 165.0, 169.0, 35.0, 183.0, 137.0, 130.0 (2C), 131.0 (2C), 136.3; anal. calcd. for C20H14ClN3O2S2: C, 56.13; H, 3.30; N, 9.82; found: C, 56.21; H, 3.28; N, 9.71; MS (EI, 70 eV): m/z (%) 427 (M+), 428 (M+1).
2-(5-((2-Phenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-bromophenyl) ethanone (5c) Yield: (70%); m.p.: 110–116 °C; 1H NMR (CDCl3, 300 MHz): δ 7.4–7.6 (m, 5H, Ar-H), 7.1 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 7.9 (d, J = 8.3 Hz, 2H, Ar-H), 7.6 (d, J = 8.3 Hz, 2H, Ar-H); anal. calcd. for C20H14BrN3O2S2: C, 50.58; H, 2.99; N, 8.90; found: C, 50.49; H, 3.11; N, 8.85; MS (EI, 70 eV): m/z (%) 471 (M+), 472 (M+1).
2-(5-((2-Phenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-phenylethanone (5d) Yield: (72%); m.p.: 100–106 °C; IR (KBr, cm−1): 3120, 2931, 1690, 785, 688; 1H NMR (CDCl3, 300 MHz): δ 7.4–7.9 (m, 10H, Ar-H), 7.1 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2); 13C NMR (75 MHz, CDCl3): δ 128.8, 129.0 (2C), 127.0 (2C), 132.0, 169.0, 109.0, 150.0. 32.0, 165.0, 169.0, 35.0, 183.0, 135.0, 131.0 (2C), 131.5 (2C), 129.3; anal. calcd. for C20H15N3O2S2: C, 61.05; H, 3.84; N, 10.68; found: C, 61.14; H, 3.74; N, 10.56; MS (EI, 70 eV): m/z (%) 393 (M+), 394 (M+1).
2-(5-((2-(4-Chlorophenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-fluoro phenyl)ethanone (5e) Yield: (69%); m.p.: 115–118 °C; 1H NMR (CDCl3, 300 MHz): δ 7.4 (d, J = 8.3 Hz, 2H, Ar-H), 7.5 (d, J = 8.3 Hz, 2H, Ar-H), 7.2 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 8.1 (d, J = 8.4 Hz, 2H, Ar-H), 7.3 (dd, J = 11.2 and 8.3 Hz, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 134.2, 128.7 (2C), 128.3 (2C), 131.0, 170.1, 109.4, 150.1, 32.0 (CH2), 166.4, 170.2, 35.0 (S-CH2), 183.1 (C=O), 132.0, 130.0 (2C), [116.2, 115.9 (d, J = 22.5 Hz, 2C)], [164.4, 161.2 (d, J = 244 Hz, 1C)]; anal. calcd. for C20H13ClFN3O2S2: C, 53.87; H, 2.94; N, 9.42; found: C, 53.79; H, 2.79; N, 9.34. MS (EI, 70 eV): m/z (%) 445 (M+), 446 (M+1).
2-(5-((2-(4-Chlorophenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-chlororophenyl) ethanone (5f) Yield: (64%); m.p.: 112–116 °C; IR (KBr, cm−1): 3090, 2950, 1690, 2835, 964, 802, 765; 1H NMR (CDCl3, 300 MHz): δ 7.4 (d, J = 8.3 Hz, 2H, Ar-H), 7.5 (d, J = 8.3 Hz, 2H, Ar-H), 7.2 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 7.8 (d, J = 8.3 Hz, 2H, Ar-H), 7.9 (d, J = 8.3 Hz, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 134.2, 128.7 (2C), 128.3 (2C), 131.0, 170.1, 109.4, 150.1, 32.0 (CH2), 166.4, 170.2, 35.0 (S-CH2), 183.1 (C=O), 135.7, 130.0 (2C), 130.8 (2C), 136.7; anal. calcd. for C20H13Cl2N3O2S2: C, 51.95; H, 2.83; N, 9.09; found: C, 52.08; H, 2.76; N, 9.18. MS (EI, 70 eV): m/z (%) 461 (M+), 462 (M+1).
2-(5-((2-(4-Chlorophenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-(4-bromo phenyl) ethanone (5g) Yield: (71%); m.p.: 101–105 °C; 1H NMR (CDCl3, 300 MHz): δ 7.4 (d, J = 8.3 Hz, 2H, Ar-H), 7.5 (d, J = 8.3 Hz, 2H, Ar-H), 7.2 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 7.8 (d, J = 8.1 Hz, 2H, Ar-H), 7.6 (d, J = 8.1 Hz, 2H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 134.2, 128.3 (2C), 128.5 (2C), 131.0, 170.0, 109.4, 150.1, 32.0 (CH2), 166.4, 170.2, 35.0 (S-CH2), 183.1 (C=O), 135.5, 131.0 (2C), 131.5 (2C), 128.0; anal. calcd. for C20H13BrClN3O2S2: C, 47.40; H, 2.59; N, 8.29; found: C, 47.31; H, 5.57; N, 8.92. MS (EI, 70 eV): m/z (%) 505 (M+), 506 (M+1).
2-(5-((2-(4-Chlorophenylthiazol-4-yl)methyl)-1,3,4-oxadiazol-2-ylthio)-1-phenyl ethanone (5h) Yield: (69%); m.p.: 121–125 °C; IR (KBr, cm−1): 3120, 2930, 1690, 933, 785, 688; 1H NMR (CDCl3, 300 MHz): δ 7.4 (d, J = 8.3 Hz, 2H, Ar-H), 7.5 (d, J = 8.3 Hz, 2H, Ar-H), 7.2 (s, 1H, thiazolyl-H), 4.4 (s, 2H, CH2), 4.8 (s, 2H, S-CH2), 7.6–7.8 (m, 5H, Ar-H); 13C NMR (75 MHz, CDCl3): δ 134.2, 128.5 (2C), 128.2 (2C), 131.1, 170.1, 109.5, 150.1, 32.2 (CH2), 166..0, 170.2, 35.0 (S-CH2), 183.1 (C=O), 135.8, 128.7 (2C), 128.5 (2C), 129.2; anal. calcd. for C20H14ClN3O2S2: C, 56.13; H, 3.30; N, 9.82; found: C, 56.27; H, 3.21; N, 9.78. MS (EI, 70 eV): m/z (%) 427 (M+), 428 (M+1).
6. Conclusions
Different S-substituted 1,3,4-oxadiazole derivatives were synthesized and evaluated for their antimicrobial activities. It was interesting to note that compounds with S-substitution were found to be biologically more potent than their respective unsubstituted derivatives. Therefore, our assumption that antimicrobial activity could be modified by incorporating more than one heterocyclic nucleus in the same molecule could possibly lead us to derivatives with enhanced activity. Thus, these molecules could act as lead molecules for further exploration of new drug molecules.
Author Contributions
Literature and spectral analysis, S.V.K.; Designing and synthesis of molecules, S.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank the G. E. Society’s HPT Arts and RYK Science College, Nashik, for providing laboratory facility. The authors also thank BCUD, Pune University and UGC, New Delhi, for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-substituted-thiazolyl)oxamic acid derivatives, a new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.N.; Xavier, F.P. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem. 2009, 24, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.W.; Cantrell, A.S. Phenethylthiazolethiourea (PETT) Compounds, a New Class of HIV-1 Reverse Transcriptase Inhibitors. 1. Synthesis and Basic Structure-Activity Relationship Studies of PETT Analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef]
- Carter, J.S.; Kramer, S.; Zweifel, B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 1171–1174. [Google Scholar] [CrossRef]
- Rudolph, J.; Geschke, F.U. Seco-Cyclothialidines: New Concise Synthesis, Inhibitory Activity toward Bacterial and Human DNA Topoisomerases, and Antibacterial Properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Antitumor Benzothiazoles. 16.1 Synthesis and Pharmaceutical Properties of Antitumor 2-(4-Aminophenyl)benzothiazole Amino Acid Prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef]
- Ojika, M.; Suzuki, Y.; Tsukamoto, A.; Sakagami, Y.; Fudou, R.; Yoshimura, T.; Yamanaka, S. Cystothiazoles A and B, new bithiazole-type antibiotics from the myxobacterium Cystobacter fuscus. J. Antibiot. 1998, 51, 275–281. [Google Scholar] [CrossRef][Green Version]
- Kalkhambkar, R.G.; Kulkarni, G.M.; Shivkumar, H.; Rao, N.R. Synthesis of novel triheterocyclic thiazoles as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2007, 42, 1272–1276. [Google Scholar] [CrossRef]
- Franklin, P.X.; Pillai, A.D.; Rathod, P.D.; Yerande, S.; Nivsarkar, M.; Padh, H.; Vasu, K.K.; Sudarsanam, V. 2-Amino-5-thiazolyl motif: A novel scaffold for designing anti-inflammatory agents of diverse structures. Eur. J. Med. Chem. 2008, 43, 129–134. [Google Scholar] [CrossRef]
- Shelke, S.H.; Mhaske, P.C.; Nandave, M.; Narkhade, S.; Walhekar, N.M.; Bobade, V.D. Synthesis and pharmacological evaluation of a novel series of 3-aryl-2-(2-substituted-4-methylthiazole-5-yl)thiazolidin-4-one as possible anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2012, 22, 6373–6376. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Ashour, H.M.A.; Ghany, Y.S.A.; Bekhit, A.E.A.; Baraka, A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zink, D.L.; Ushio, M.; Burgess, B.; Onishi, R.; Masurekar, P.; Barrett, J.F.; Singh, S.B. Isolation, structure, and antibacterial activity of thiazomycin A, a potent thiazolyl peptide antibiotic from Amycolatopsis fastidiosa. Bioorg. Med. Chem. 2008, 16, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.H.; Mhaske, P.C.; Hande, P.; Bobade, V.D. Synthesis and Antimicrobial Activities of Novel Series of 1-((4-Methyl-2-Substituted Thiazol-5-yl)Methyleneam INO)-2-Substituted Isothiourea Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1262–1270. [Google Scholar] [CrossRef]
- Shiradkar, M.R.; Murahari, K.K.; Gangadasu, H.R.; Tatikonda, S.; Chakravarthy, A.K.; Dolly, P.; Kaur, R.; Burange, P.; Ghogare, J.; Mokalec, V.; et al. Synthesis of new S-derivatives of clubbed triazolyl thiazole as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. 2007, 15, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Macaev, F.; Rusu, G.; Pogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure-anti-mycobacterial activities. Bio-Org. Med. Chem. 2005, 13, 4842–4850. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, G.T.; Ozdemir, A.; Kaplancikli, Z.A.; Benkli, K.; Chevallet, P.; Akalin, G. Synthesis and antituberculosis activity of new thiazolylhydrazone derivatives. Eur. J. Med. Chem. 2008, 43, 981–985. [Google Scholar] [CrossRef]
- Li, Z.; Khaliq, M.; Zhou, Z.; Post, C.B.; Kuhn, R.J.; Cushman, M. Design, Synthesis, and Biological Evaluation of Antiviral Agents Targeting Flavivirus Envelope Proteins. J. Med. Chem. 2008, 51, 4660–4671. [Google Scholar] [CrossRef]
- Rudolph, J.; Chen, L.; Majumdar, D.; Bullock, W.H.; Burns, M.; Claus, T.; Dela Cruz, F.E.; Daly, M.; Ehrgott, F.J.; Johnson, J.S.; et al. Indanylacetic Acid Derivatives Carrying 4-Thiazolyl-phenoxy Tail Groups, a New Class of Potent PPAR α/γ/δ Pan Agonists: Synthesis, Structure−Activity Relationship, and In Vivo Efficacy. J. Med. Chem. 2007, 50, 984–1000. [Google Scholar] [CrossRef]
- Siddiqui, N.; Arshad, M.F.; Ahsan, W. Thiazoles: A valuable insight into the recent advances and biological activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar]
- Vasu, N.; Goud, B.B.; Kumari, Y.B.; Rajitha, B. Design, synthesis and biological evaluation of some novel benimidazole based thiazolyl amines. Rasayan. J. Chem. 2013, 6, 201–206. [Google Scholar]
- Singh, N.; Bhati, S.K.; Kumar, A. Thiazolyl/oxazolyl formazanyl indoles as potent anti-inflammatory agents. Eur. J. Med. Chem. 2008, 43, 2597–2609. [Google Scholar] [CrossRef] [PubMed]
- Luzina, E.L.; Popov, A.V. Synthesis and anticancer activity of N-bis(trifluoromethyl)alkyl-N’-thiazolyl and N-bis(trifluoromethyl)alkyl-N’-benzothiazolyl ureas. Eur. J. Med. Chem. 2009, 44, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.K.; Tripathi, R.; Katti, S.B.; Pannecouque, C.; Clercq, E. Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem. 2008, 43, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Nagatomi, Y.; Hirata, Y.; Ito, S.; Suzuki, G.; Kimura, T.; Maehara, S.; Hikichi, H.; Satow, A.; Hata, M.; et al. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 2009, 19, 5464–5468. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009, 44, 3627–3636. [Google Scholar] [CrossRef]
- Mullican, M.D.; Wilson, M.W.; Connor, D.T. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef]
- Zareef, M.; Iqbal, R.; Al-Masoudi, N.A. Synthesis, Anti–HIV, and Antifungal Activity of New Benzensulfonamides Bearing the 2,5-Disubstituted-1,3,4-Oxadiazole Moiety. Phosphorus Sulfur Silicon 2007, 182, 281–298. [Google Scholar] [CrossRef]
- Mulvad, V.V.; Chaskar, A.C. Synthesis and antibacterial activity of new oxadiazolo[1,3,5]-triazine 1,2,4-triazolo 1,3,4-oxadiazole derivatives. Ind. J. Chem. 2006, 45B, 1710–1715. [Google Scholar]
- Kambale, R.R.; Sudha, B.S. Synthesis and pharmacological screening of 5-methyl-3-[P-(6′-aryl-2′-thioxo-1′,2′,5′,6′-tetrahydro-pyrimidin-4′-yl)-phenyl]-3 H-2-oxo-D4-1,3,4-oxadiazoles. Ind. J. Pharm. Sci. 2006, 68, 249–253. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Vargas-Villarreal, J. Synthesis and antimycobacterial activity of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines. J. Bioorg. Med. Chem. 2007, 15, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.V.; Bothara, K.G. Design, Synthesis and Evaluation of Antiinflammatory, Analgesic and Ulcerogenicity studies of Novel S-Substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of Diclofenac acid as Nonulcerogenic Derivatives. Bioorg. Med. Chem. 2008, 16, 1822–1831. [Google Scholar] [CrossRef]
- Zarghi, A.; Faizi, M.; Shafaghi, B. Design and synthesis of new 2-substituted-5-(2-benzylthiophenyl)-1,3,4-oxadiazoles as benzodiazepine receptor agonists. Bioorg. Med. Chem. Lett. 2005, 15, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, S.G.; Jiang, L.L.; Hao, G.F.; Wang, Z.F.; Wu, Q.Y.; Xi, Z.; Yang, G.F. Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorg. Med. Chem. 2012, 20, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.S.; Rajendraprasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M.; Kistayya, C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010, 45, 2063–2074. [Google Scholar] [CrossRef]
- Bondock, S.; Adel, S.; Etman, H.A.; Badria, F.A. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012, 48, 192–199. [Google Scholar] [CrossRef]
- Mhaske, P.C.; Shelke, S.H.; Bhoye, M.; Bobade, V.D. Synthesis and Antimicrobial Screening of 2-Aryl-5-((2-arylthiazol-4-yl)methyl)-1,3,4-oxadiazole Derivatives. J. Het. Chem. 2017, 54, 1590–1597. [Google Scholar] [CrossRef]
- Yeeman, K.R.; Lei, Z. Synthesis and biological evaluation of 2,4-diaminoquinazoline derivatives as novel heat shock protein 90 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1593–1597. [Google Scholar]
- Mallikarjuna, B.P.; Sastry, B.S. Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxadiazole ring systems—A novel class of potential antibacterial, antifungal and antitubercular agents. Eur. J. Med. Chem. 2009, 44, 4739–4746. [Google Scholar] [CrossRef]
- Kiselyov, A.S.; Semenova, M.N. Novel derivatives of 1,3,4-oxadiazoles are potent mitostatic agents featuring strong microtubule depolymerizing activity in the sea urchin embryo and cell culture assays. Eur. J. Med. Chem. 2010, 45, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Mruthynjayswami, B.H.M.; Basararajaiah, S.M.B. Synthesis and antimicrobial activity of novel ethyl-5-(ethoxycarbonyl)-4-methyl thiazol-2-yl carbamate compounds. Ind. J. Chem. 2009, 48B, 1274–1278. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).