Abstract
In search of new thermotropic, photoswitchable materials, a number of 4′-alkoxy-4-(ω-cinnamoylalkoxy)azobenzenes were prepared. The synthetic procedure included O-alkylation of 4-nitrophenol, followed by reduction of the nitro group (H2, Pd/C), diazotization of the aniline, and subsequent reaction with ω-hydroxyalkoxybenzenes, followed by a modified Appel-type esterification (BrCCl3, PPh3). The photochemical behavior of the substances was investigated.
1. Introduction
Photoswitchable molecules are able to isomerize between at least two metastable forms when photoirradiated [1]. These types of molecules have found interest in different areas in physics, chemistry, and biology [2,3,4,5]. Photoswitchable molecules have a wide range of applications, which include their use in photoelectric cells. They are also utilized in the generation of three-dimensional animations and images, as well as in screen displays in conjunction with liquid crystals [6]. Photoswitching molecules can be used as dopants in liquid crystalline hosts. Alternatively, photoswitching compounds can be liquid crystalline themselves, where often the photoswitching unit is an azobenzene. Thus, azobenzene derivatives have been utilized in photoresponsive functional devices in smart polymers [7], in molecular switches [8], in data storage systems [9], and as molecular “machines” in supramolecular organic chemistry [10,11,12]. In this regard, 4′-alkoxy-4-(ω-cinnamoylalkoxy)azobenzenes of type 1 (Figure 1) were prepared as potentially photoswitchable compounds with the azo- and cinnamoyl functions as two photoreactive groups [13].
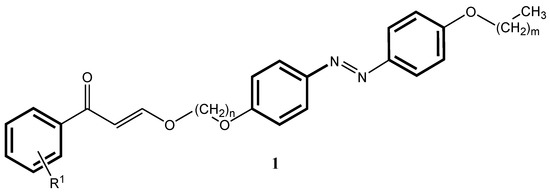
Figure 1.
4′-alkoxy-4-(ω-cinnamoylalkoxy)azobenzene (1).
2. Experimental Part
General. The compounds were synthesized, purified (using crystallization or column chromatography), and characterized by 1H NMR, 13C NMR, DEPT, and/or INEPT techniques, LC-MS-MS, and IR spectroscopy. Selected compounds were analyzed by UV–Vis spectroscopy and submitted to DSC thermal analysis and X-ray single-crystal structural determination. Selected azo-cinnamates were photoirradiated in an attempt to photo-isomerize the molecules. The progress of the photoreactions was followed by either UV–Vis or 1H NMR spectroscopy.
Column chromatography was carried out on commercial 60 Å silica gel (230–400 mesh, Merck grade 9385, Sigma-Aldrich) and on recycled silica gel. Analytical thin-layer chromatography (TLC) was carried out on TLC-Alu-foils from Fluka (with a fluorescent indicator at λ = 254 nm). 1H NMR (at 400 MHz) and 13C NMR (at 100.5 MHz) spectra were taken on a Varian 400 MHz spectrometer. Infrared spectra were taken on a Thermo Nicolet Nexus 670 FT-IR spectrometer (solid samples as KBr pellets). UV–Vis spectroscopy was performed on a UV-1800 (Shimadzu) spectrophotometer. For photoirradiation experiments, a Luzchem LZC 4V photoreactor was used with either 13 USHIO G8T5 lamps (7.2 W low-pressure mercury arc lamps with a radiation peak at λ = 253.7 nm) or with 14 Hitachi FL8BL-B (0.75 W, UV irradiance 8.0 (µ/cm)2, with a radiation peak at λ = 352 nm). CH2Cl2 (Sigma-Aldrich, purris. pa, ≥99.9% (GC)) and benzene were used as solvents in the photoirradiation experiments. Mass spectrometry on the synthesized compounds was performed using a LC-MS-MS 8060 (Shimadzu) with Dr. Iltaf Khan.
Synthesis of (E)-11-(4-((E)-(4-(Octyloxy)phenyl)diazenyl)phenoxy)undecyl 3-(4-methoxyphenyl)-acrylate (1a) by Modified Appel Reaction:
To a solution of triphenylphosphine (PPh3, 970 mg, 3.70 mmol) in dry CH2Cl2, (15 mL) bromotrichloromethane (BrCCl3, 720 mg, 3.63 mmol) was added dropwise, and the resulting solution was stirred at reflux temperature for 25 min, during which it turned yellow-brown. Thereafter, 3-(4-methoxyphenyl)acrylic acid (9a, 530 mg, 3.00 mmol) was added, and the mixture was stirred at reflux temperature for 30 min. Then, 8a (600 mg, 1.21 mmol) was added, and the mixture was stirred at reflux temperature for an additional 14 h. The cooled solution was submitted directly to column chromatography on silica gel (CH2Cl2) to give 1a (625 mg, 78.5%) as a yellow solid (Figure 2).
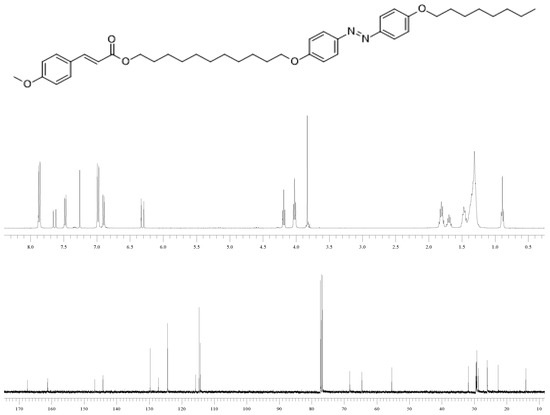
Figure 2.
1H NMR and 13C NMR spectral data of (E)-11-(4-((E)-(4-(octyloxy)phenyl)diazenyl)phenoxy)undecyl 3-(4-methoxyphenyl)-acrylate (1a).
δH (400 MHz, CDCl3): 0.88 (3H, t, CH3, 3J = 6.8 Hz), 1.24-1.84 (24H, m, CH2), 3.83 (3H, s, OCH3), 4.02 (4H, t, OCH2, 3J = 6.7 Hz), 4.18 (2H, t, OCH2, 3J = 6.5 Hz), 6.31 (1H, d, CH, 3J = 16.0 Hz), 6.89 (2H, d, CH, 3J = 8.0 Hz), 6.98 (4H, d, CH, 3J = 8.0 Hz), 7.47 (2H, d, CH, 3J = 8.0 Hz), 7.63 (1H, d, CH, 3J = 16.0 Hz), 7.86 (4H, d, CH, 3J = 8.0 Hz); δC (100.5 MHz, CDCl3): 14.1 (CH3), 22.6 (CH2), 25.9 (CH2), 26.0 (CH2), 28.7 (CH2), 29.2 (CH2), 29.2 (CH2), 29.2 (CH2), 29.2 (CH2) 29.3 (CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2), 31.8 (CH2), 55.3 (OCH2), 68.2 (OCH2), 68.2 (OCH2), 68.3 (OCH2), 114.2 (CH), 114.6 (CH), 115.7 (CH), 124.3 (CH), 127.1 (CH), 129.6 (CH), 144.2 (CH), 146.7 (CH), 161.1 (CH), 161.2 (CH), 167.4 (C=O); υ IR (KBr, cm−1): 3429, 2029, 2851, 1702, 1632, 1602, 1580, 1514, 1496, 1473, 1289, 1243, 117, 1027, 842. Mass found: 657.
3. Results and Discussion
3.1. Synthesis of the Target Compounds
The syntheses of target compound 1 started with commercially available 4-nitrophenol (2), which was subjected to a Williamson ether synthesis with various, commercially available ω-bromoalkan-1-ols (3). With the relatively acidic phenol system, K2CO3 can be used as a base. Some of the products 4 were gained by simple extraction; some of the products needed to be purified by column chromatography on silica gel. Next, the nitro group in 4 needed to be reduced. There are various ways to reduce nitrobenzenes to anilines, such as with low valent metals, zinc, and tin, in an acidic medium [14]. Also, a reduction with samarium is possible [15]. Efficient, however, is the hydrogenation of nitrobenzenes over metal catalysts as little solid waste is created as a side product. Typical metal catalysts for this reaction are Raney Nickel [16], finely divided nickel on solid [17], also in the form of Urushibara nickel, as well as platinum oxide PtO2. Also, palladium on carbon can be used as hydrogenation catalyst. The hydrogenation of compounds 4 over 10w% Pd/C in THF using externally supplied hydrogen was successful, and anilines 5 were produced almost quantitatively. No extensive purification of the products was necessary. Because of safety concerns, later NaBH4–acetic acid was used as an internal hydrogen source [18]. These reactions, however, were very slow to complete at the reaction scale used. Next, the obtained anilines 5 were subjected to a diazotization (NaNO2, HCl) in the presence of phenol (6) to give diazobenzenes 7. The reaction was not easy to perform, and reaction yields varied. The diazobenzenes 7 were alkylated at the phenol OH using K2CO3 as base. The base was not strong enough to also deprotonate the alcohol function in 7, so that the alkylation proceeded at the phenolic OH only, giving products 8, although the reaction temperature needed was quite high (120 °C). For the preparation of azo-cinnamates 1, the final step was an esterification reaction. For this, we decided to generate cinnamoyl halides in situ, via an Appel reaction, utilizing the system BrCCl3, PPh3, Et3N) [19], which were then reacted with 8 (Scheme 1).
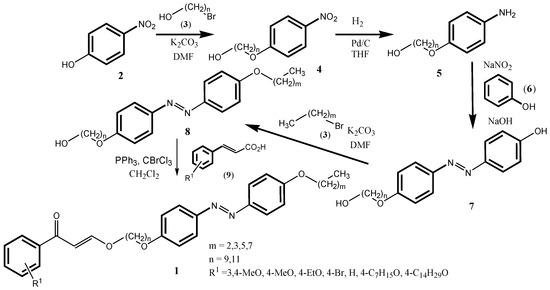
Scheme 1.
Synthesis of the Target Compounds.
3.2. Photoirradiation of the 4′-Alkoxy-4-(ω-cinnamoylalkoxy)azobenzenes
On the UV spectrum, the 4′-alkoxy-4-(ω-cinnamoylalkoxy)azobenzenes 1, prepared above, show well-separated absorption maxima, with the absorption of the azobenzene unit at λ = 360 nm, and a variable absorption of cinnamate moiety of λ = 259–325 nm, depending on the substitution pattern of the cinnamate (Figure 3). The main idea was to trigger only the azo group in the synthesized azo-cinnamates 1, while leaving the trans-double bond of the cinnamate unit untouched by irradiating the molecules at λ = 350 nm, close to the absorption maximum of the azobenzene moiety at λ = 360 nm. The photoisomerization experiments were carried out with the same sample concentrations for all compounds (1.0 × 10−5 mol/L). The experiments were followed by using UV spectroscopy and 1H NMR spectroscopy (Figure 4). The results proved that the photoisomerization time depends on the terminal substitution and different carbon chains linked between the moieties of the molecules although all molecules reached a photostationary phase within 35 s of photoirradiation. For all compounds, with increases in the radiation time, the absorption peak around λ = 360 nm started to decrease (trans-form), while the peak around λ = 450 nm increased (cis-form). After the photoirradiation was stopped, a slow, thermally driven cis–trans isomerization took place, the speed of which, again, depended on the terminal substitution pattern and carbon chain lengths within the molecules. Here, for some molecules, the thermal cis–trans conversion was not totally complete, even after 25 h, where all experiments were carried out at room temperature.
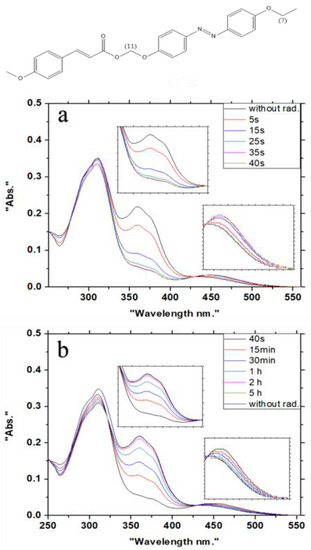
Figure 3.
UV spectra of 1b: (a) during irradiation at λ = 350 nm (photochemical trans–cis isomerization of the azobenzene moiety), (b) thermal cis–trans isomerization of the azobenzene moiety.
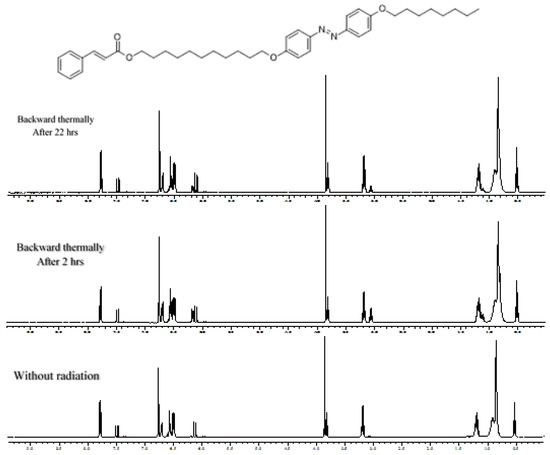
Figure 4.
Monitoring by 1H NMR spectroscopy the thermal cis–trans isomerization of a mixture of cis–/trans–1c after photoirradiation of trans–1c. The azobenzene unit isomerizes, while the cinnamate moiety remains trans-configurated during the initial photoisomerization process.
4. Conclusions
A number of 4′-alkoxy-4-(ω-cinnamoylalkoxy)azobenzenes were prepared. It was found that the azobenzene unit and the cinnamate moiety in these molecules absorb at different wavelengths and can be addressed selectively by photoirradiation. The azo-unit was trans/cis isomerized photochemically, while the cinnamate moiety remained trans-configurated. After the irradiation was halted, the compounds cis/trans isomerized thermally. The time the molecules reached photochemical equilibrium depended on the terminal substitution pattern and carbon chain lengths within the molecules. Some of the compounds have been found to exhibit narrow mesophases, so their thermotropic behavior needs to be studied in greater detail. It is expected that the molecules can also be used as switchable dopants in liquid crystalline hosts.
Author Contributions
Methodology, investigation, data curation, A.A.-H.; Conceptualization; writing—original draft preparation, supervision, project administration T.T.; writing—review and editing, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Additional data can be obtained from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene photoswitching without ultraviolet light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef] [PubMed]
- Heckel, A.; Mayer, G. Light-responsive nucleic acids for the spatiotemporal control of biological processes. In The Chemical Biology of Nucleic Acids; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 279–306. [Google Scholar] [CrossRef]
- Russew, M.-M.; Hecht, S. Photoswitches: From molecules to materials. Adv. Mat. 2010, 22, 3348–3360. [Google Scholar] [CrossRef] [PubMed]
- Szobota, S.; Isacoff, E.Y. Optical control of neuronal activity. Ann. Rev. Biophys. 2010, 39, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Wyart, C.; Bene, F.D.; Warp, E.; Scott, E.K.; Trauner, D.; Baier, H.; Isacoff, E.Y. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 2009, 461, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Stranius, K.; Börjesson, K. Determining the photoisomerization quantum yield of photoswitchable molecules in solution and in the solid state. Sci. Rep. 2017, 7, 41145. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Nakashima, Y.; Irie, M. A novel photoresponsive π-conjugated polymer based on diarylethene and its photoswitching effect in electrical conductivity. Adv. Mat. 2005, 17, 309–314. [Google Scholar] [CrossRef]
- Yasuda, S.; Nakamura, T.; Matsumoto, M.; Shigekawa, H. Phase switching of a single isomeric molecule and associated characteristic rectification. J. Am. Chem. Soc. 2003, 125, 16430–16433. [Google Scholar] [CrossRef] [PubMed]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. A 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Broichhagen, J.; Trauner, D. The in vivo chemistry of photoswitched tethered ligands. Curr. Opin. Chem. Biol. 2014, 21, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Goulet-Hanssens, A.; Barrett, C.J. Photo-control of biological systems with azobenzene polymers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3058–3070. [Google Scholar] [CrossRef]
- part of Al-Hemyari, A. Preparation and Characterization of Novel Azocinnamates and Steroidal azo Ethers. Masters’ Thesis, United Arab Emirates University, Al Ain, United Arab Emirates, 2019. [Google Scholar]
- Faul, M.M.; Thiel, O.R. Tin(II) Chloride. In Encyclopedia of Reagents for Organic Synthesis. e-EROS 2005. [Google Scholar] [CrossRef]
- Basu, M.K.; Becker, F.F.; Banik, B.K. Ultrasound-promoted highly efficient reduction of aromatic nitro compounds to the aromatic amines by samarium/ammonium chloride. Tetrahedron Lett. 2000, 41, 5603–5606. [Google Scholar] [CrossRef]
- Allen, C.F.H.; VanAllan, J. 2-Amino-p-cymene: Carvacrylamine. Org. Synth. 1942, 22, 9. [Google Scholar] [CrossRef]
- Mazaheri, O.; Kalbasi, R.J. Preparation and characterization of Ni/mZSM-5 zeolite with a hierarchical pore structure by using KIT-6 as silica template: An efficient bi-functional catalyst for the reduction of nitro aromatic compounds. RSC Adv. 2015, 5, 34398–34414. [Google Scholar] [CrossRef]
- Soom, N.; Thiemann, T. Hydrogenation of alkenes with NaBH4, CH3CO2H, Pd/C in the presence of O- and N-benzyl functions. Int. J. Org. Chem. 2016, 6, 1–11. [Google Scholar] [CrossRef][Green Version]
- Al-Azani, M.; al-Sulaibi, M.; al Soom, N.; Al Jasem, Y.; Bugenhagen, B.; Al Hindawi, B.; Thiemann, T. The use of BrCCl3-PPh3 in Appel type transformations to esters, O-acyloximes, amides, and acid anhydrides. Comptes Rendus Chim. 2016, 19, 921–932. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).