Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Small Unilamellar Vesicles (SUVs)
2.3. Spectroscopy
2.4. Microbial Toxicity
2.5. Confocal Laser Scanning Microscopy
3. Results and Discussion
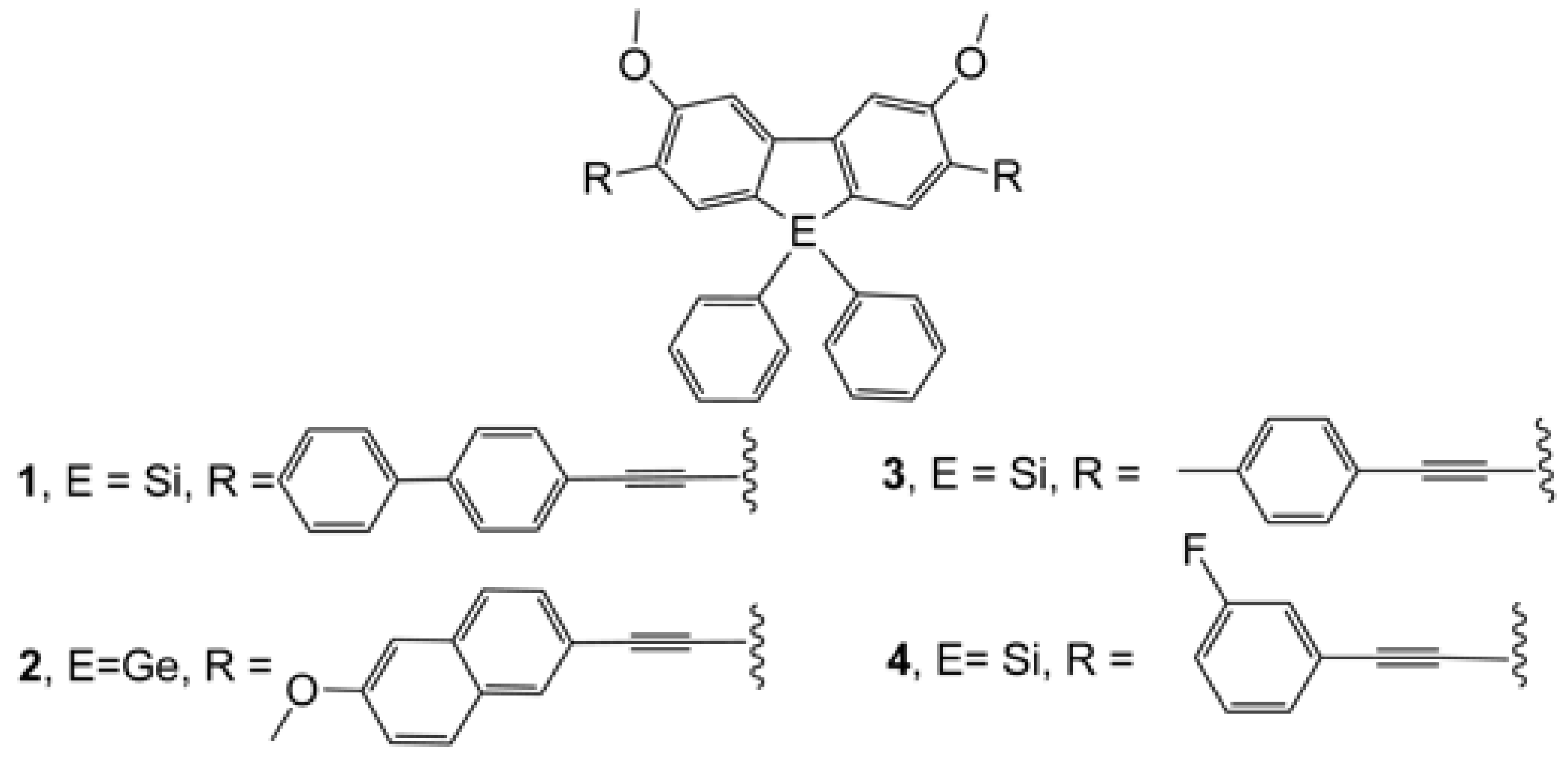
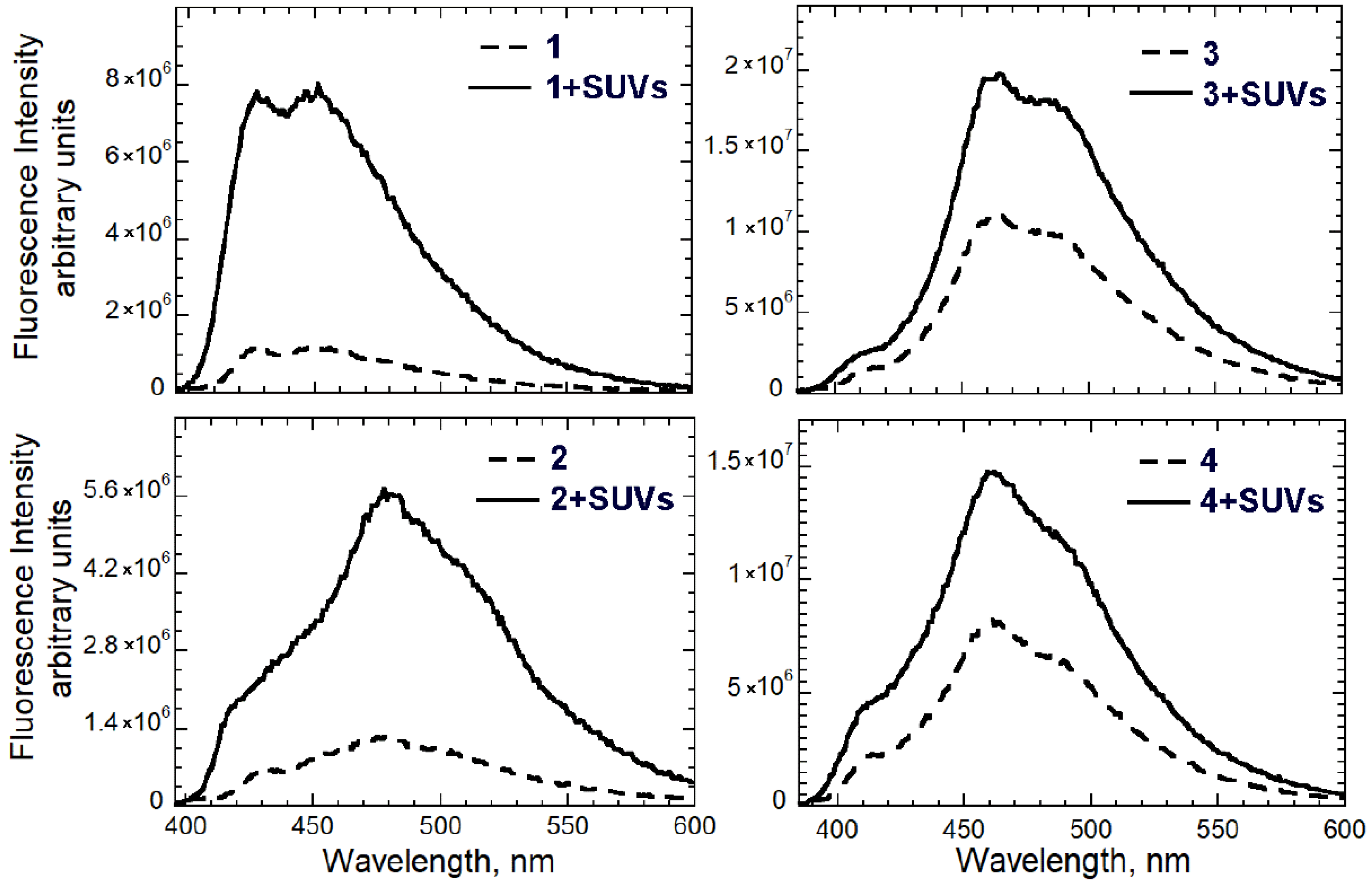
3.1. Spectroscopic Studies
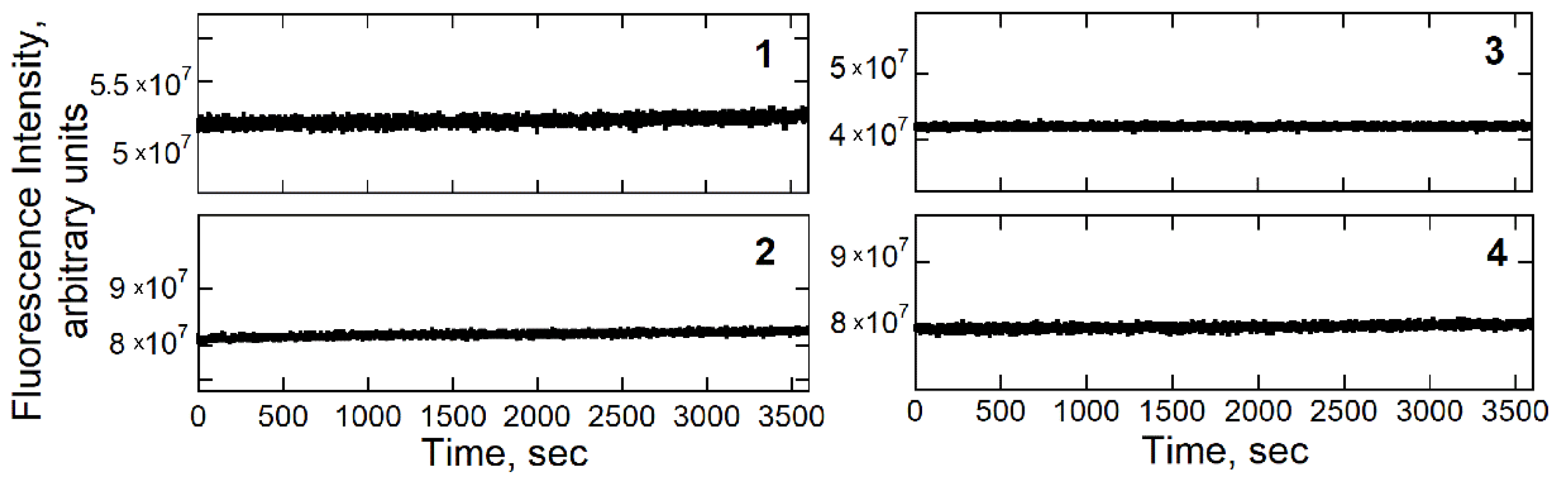
3.2. Microbial Toxicity Studies
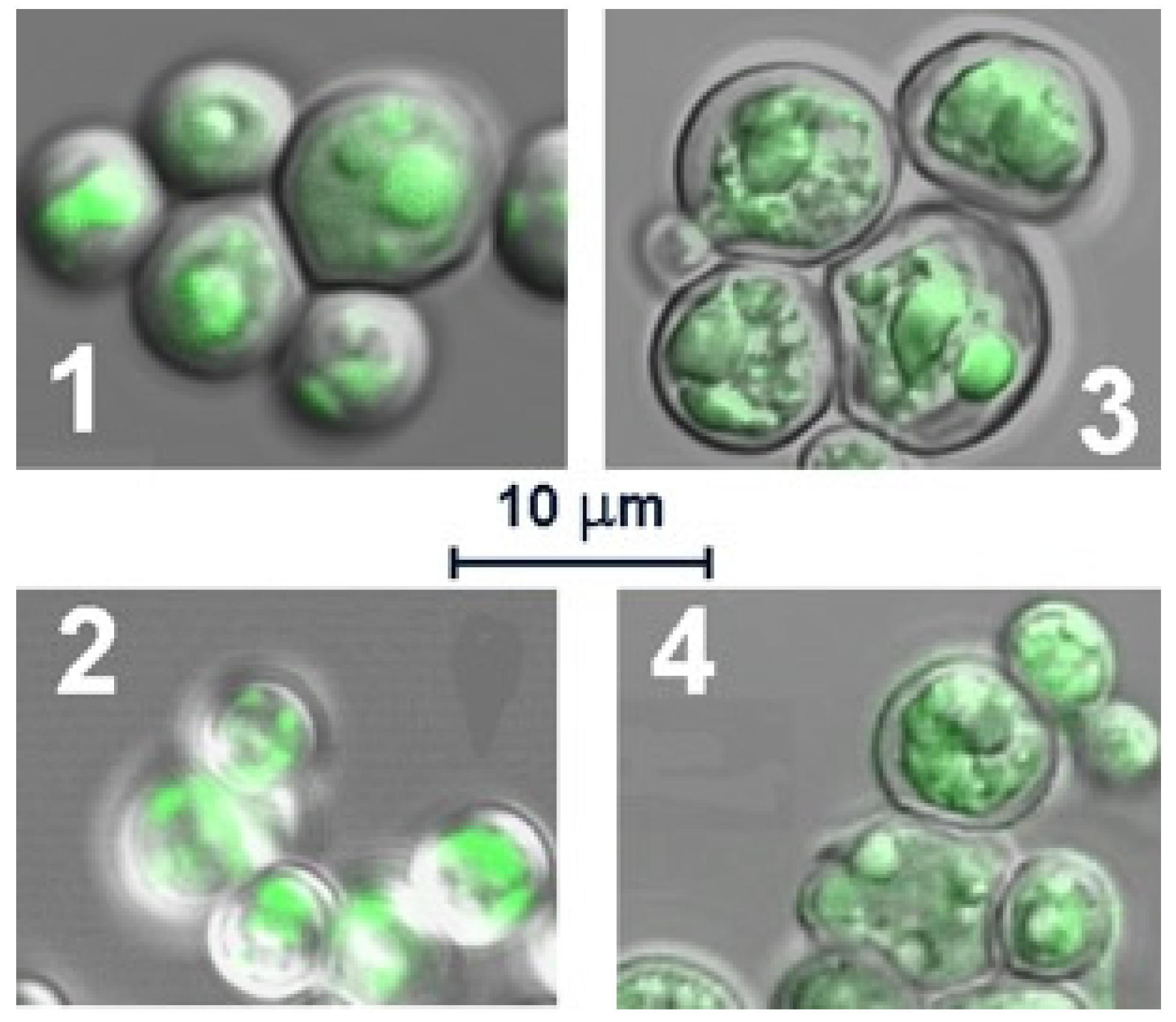
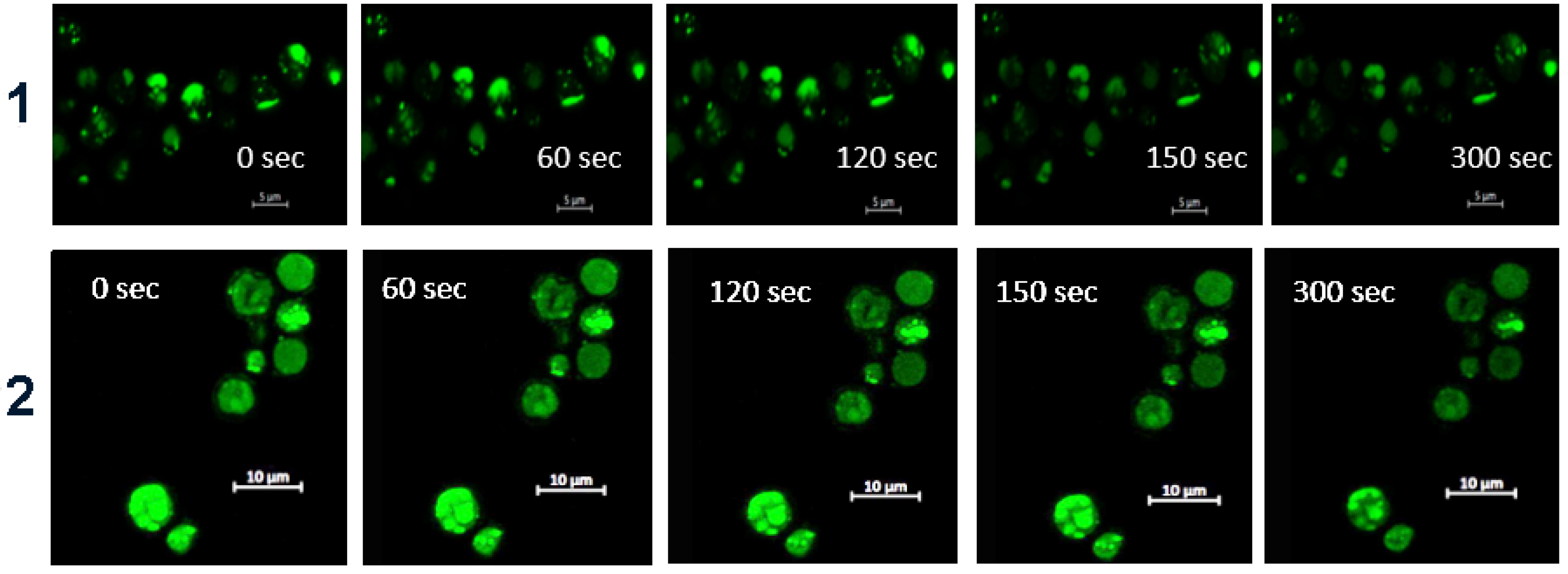
3.3. Imaging of S. cerevisiae with Metallafluorenes
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Carravilla, P.; Nieva, J.L.; Eggeling, C. Fluorescence Microscopy of the HIV-1 Envelope. Viruses 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoka, A.H.; Ashokkumar, P.; Kovtun, Y.P.; Klymchenko, A.S. Solvatochromic Near-Infrared Probe for Polarity Mapping of Biomembranes and Lipid Droplets in Cells under Stress. J. Phys. Chem. Lett. 2019, 10, 2414–2421. [Google Scholar] [CrossRef] [Green Version]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klymchenko, A.S.; Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shynkar, V.V.; Klymchenko, A.S.; Kunzelmann, C.; Duportail, G.; Muller, C.D.; Demchenko, A.P.; Freyssinet, J.M.; Mely, Y. Fluorescent biomembrane probe for ratiometric detection of apoptosis. J. Am. Chem. Soc. 2007, 129, 2187–2193. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Synthesis and characterization of luminescent 2,7-disubstituted silafluorenes. J. Organomet. Chem. 2016, 813, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Luminescent 2,7-disubstituted germafluorenes. J. Organomet. Chem. 2017, 830, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes as Sensitive Luminescent Probes of Surfactants in Aqueous Solution. J. Fluoresc. 2021, 31, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.; Filipe, H.A.; Ramalho, J.P.; Hof, M.; Loura, L.M.S. Fluorescence of nitrobenzoxadiazole (NBD)-labeled lipids in model membranes is connected not to lipid mobility but to probe location. Phys. Chem. Chem. Phys. 2016, 18, 7042–7054. [Google Scholar] [CrossRef] [PubMed]
- Mazeres, S.; Joly, E.; Lopez, A.; Tardin, C. Characterization of M-laurdan, a versatile probe to explore order in lipid membranes. F1000Research 2014, 3, 172. [Google Scholar] [CrossRef] [PubMed]
- Shaya, J.; Collot, M.; Benailly, F.; Mahmoud, N.; Mely, Y.; Michel, B.Y.; Klymchenko, A.S.; Burger, A. Turn-on Fluorene Push-Pull Probes with High Brightness and Photostability for Visualizing Lipid Order in Biomembranes. ACS Chem. Biol. 2017, 12, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Olivas, W.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging. Chem. Proc. 2021, 5, 83. https://doi.org/10.3390/CSAC2021-10455
Spikes HJ, Jarrett-Noland SJ, Germann SM, Olivas W, Braddock-Wilking J, Dupureur CM. Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging. Chemistry Proceedings. 2021; 5(1):83. https://doi.org/10.3390/CSAC2021-10455
Chicago/Turabian StyleSpikes, Helena J., Shelby J. Jarrett-Noland, Stephan M. Germann, Wendy Olivas, Janet Braddock-Wilking, and Cynthia M. Dupureur. 2021. "Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging" Chemistry Proceedings 5, no. 1: 83. https://doi.org/10.3390/CSAC2021-10455
APA StyleSpikes, H. J., Jarrett-Noland, S. J., Germann, S. M., Olivas, W., Braddock-Wilking, J., & Dupureur, C. M. (2021). Group 14 Metallafluorenes for Lipid Structure Detection and Cellular Imaging. Chemistry Proceedings, 5(1), 83. https://doi.org/10.3390/CSAC2021-10455





