1. Introduction
High temperature gas sensors are mainly designed to solve gas detection and monitoring problems with a high operating temperature environment, such as gas turbine, nuclear power plants and automobile internal combustion engine emission [
1]. As far as target gases are concerned NOx (NO
2, NO) and H
2 are among the most important. NOx is a severely toxic gas with a pungent odor arising mostly from the related human activities involving high temperature such as combustion of coal and oil at electric power plants, combustion of chemical plant and also in emissions from automotive and aircraft engines. NOx-emission leads to harmful effects on the environment and health. There is an urgent need to develop sensor control systems for exhaust emission gases to directly monitor NOx at temperatures in the range of 400–900 °C [
2]. As far as hydrogen is concerned, it is the best candidate to replace the hydrocarbon-based fuels used in many combustion engines such as those in automobiles and aircraft, which are responsible for much of today’s air pollution [
3]. Hydrogen seems to be a green, renewable energy carrier that can help solve the problems of non-sustainable energy use (fossil fuels). However, the efficient application of hydrogen requires careful consideration of the relevant safety concern. In fact, its physico-chemical properties make hydrogen a highly explosive gas [
3,
4]. Moreover, as hydrogen is colorless, odorless and tasteless, the ability to detect a hydrogen leak by means of selective sensors is highly desired.
Chemiresistive gas sensors based on semiconductor metal oxides have been drawing more and more attention because of their advantages such as low cost, lightweight, fast response/recovery times and high compatibility with microelectronic processing. Graphene and its derivatives, as well as some organic semiconductor-based materials constitute also an interesting family of chemo-resistive gas sensor due to their large surface area, good electrical, thermal and mechanical properties [
5,
6].
Cost effective metal oxide-based gas sensors such as SnO
2, WO
3, ZnO, NiO or CuO operate mostly at temperatures below 400 °C [
7,
8,
9,
10]. There are only few reports in literature focusing on their gas sensing above 400 °C. TiO
2 is one of them to be capable of operating above 500 °C. The additional benefits of TiO
2 are non-toxicity, easy fabrication, and the good chemical stability [
11]. However, TiO
2 is a high resistive n-type semiconductor with relatively poor conductivity for sensing oxidative gases such as NO
2. This disadvantage was previously reported to be overcome through addition of low valence dopant atoms which alter the electronic structure [
12,
13,
14,
15]. Another strategy is to use catalytically doped perovskite-based titanium compounds such as BaTiO
3. In this work, we report the synthesis of Co-doped TiO
2, Ni doped TiO
2 and Rh-doped BaTiO
3 by co-precipitation method and demonstrate gas sensing ability toward NO
2, NO and H
2 above 500 °C. Our results yield that Co-doping of TiO
2 promotes p-type behavior exhibiting good sensing properties to NO
2 while Ni-doping displays the maintenance of n-type behavior and better H
2-sensing properties at 600 °C. More interestingly, Rh-doped BaTiO
3 shows excellent NO sensing properties even at 900 °C.
3. Results and Discussion
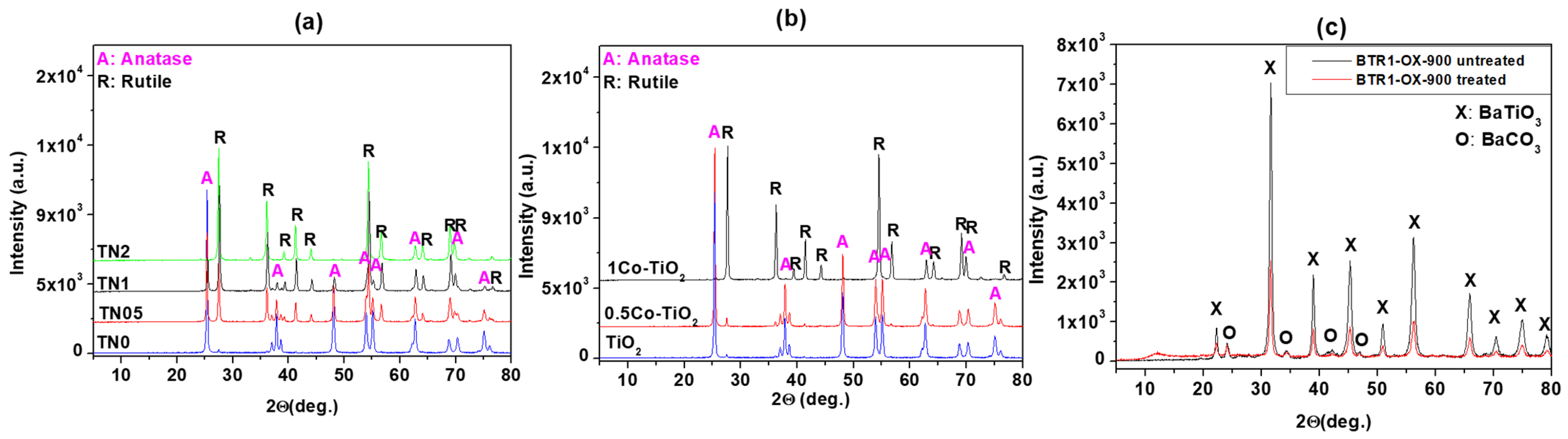
The phase identification derived from powder XRD analysis is presented on
Figure 1. As can be observed in
Figure 1a for Ni-doped TiO
2, the results indicate that anatase is present in those from undoped to 1% Ni-doped TiO
2, while rutile is the only phase present in the 2% Ni-doped TiO
2 powders. The amount of anatase (JCPDS 21-1272) decreases as the amount of nickel increases while the amount of rutile (JCPDS 21-1276) follows the opposite trend. A trace amount of ilmenite, NiTiO
3 is observed at 32.5° in the TN1 and TN2 samples (JCPDS 33-0960). These results indicate that Ni promotes the transition of anatase to rutile. As far as Co-doped TiO
2 is concerned, the results depicted on
Figure 1b reveal that, the undoped and the 0.5Co-doped TiO
2 samples showed pure TiO
2 consisted of its two polymorphs; anatase (majority) and rutile phases (minority). No other phase containing Co was observed. The 1Co-doped TiO
2 sample showed only single phase TiO
2 but this time the rutile polymorph was the major phase as anatase phase was in trace amount. The X-ray results indicate that the cobalt dopant promotes also the anatase-to-rutile phase conversion of TiO
2 but not progressively as nickel. The results of XRD analysis performed on the Rh-doped BaTiO
3 (before and after the activation) are presented in
Figure 1c. As shown, the major phase is BaTiO
3 (according to JCPDS 075-0462) and a trace amount of BaCO
3 phase is observed. No evidence of an Rh phase was encountered indicating the substitution of Ti by Rh.
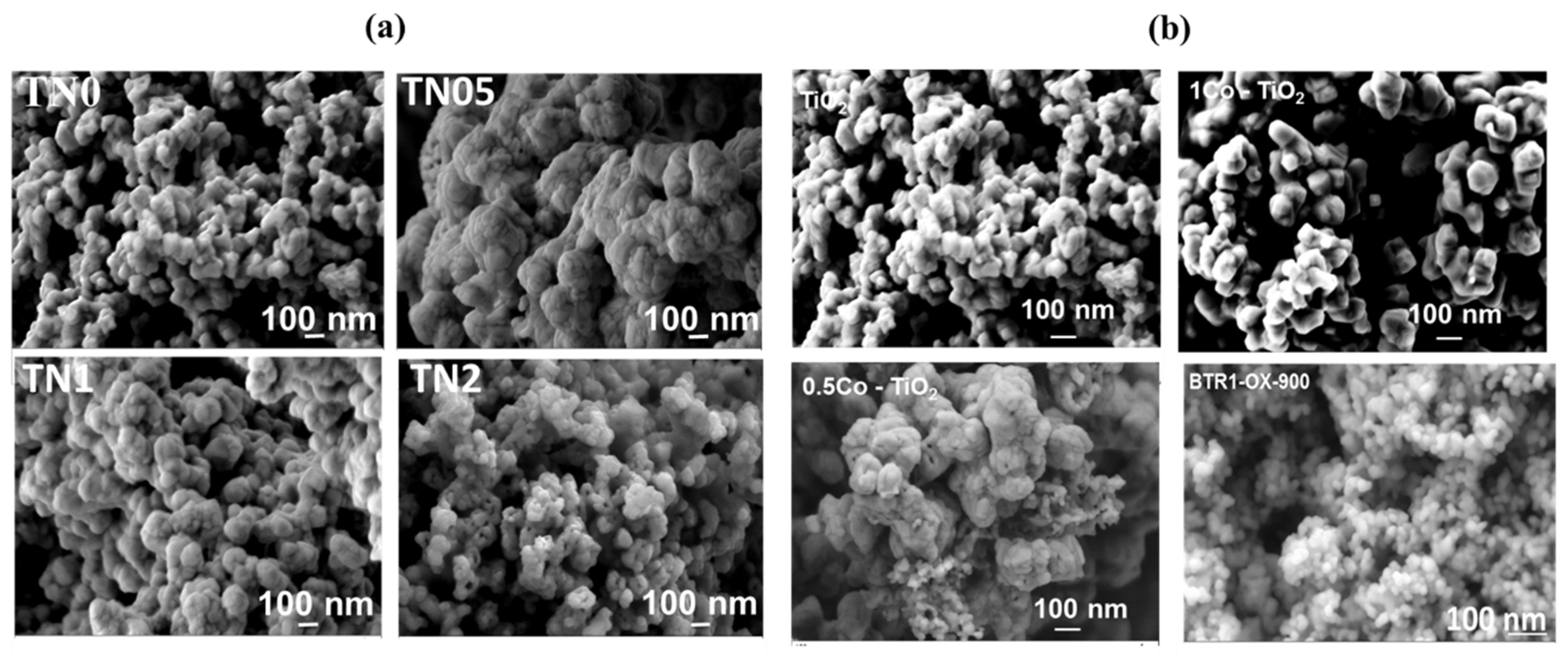
Figure 2 shows the morphology of the synthesized powders investigated by SEM. Concerning Ni-doped TiO
2 (
Figure 2a), as far as the undoped powder sample is concerned, the microstructural investigation reveals spherical nanoparticles with sizes around 70 nm, which tend to agglomerate. While the TN05 sample shows more agglomerated spherical particles, the TN1 and TN2 samples show less agglomeration with the appearance of small pores and particle size reduction for the TN2 samples.
Figure 2b shows the morphology of the Co-doped TiO
2 synthetized powders. The SEM investigation revealed the formation of spherical nanoparticles with sizes around 70 nm. As the sample 0.5Co-doped TiO
2 shows more agglomerated spherical particles, the sample 1Co-doped TiO
2 presented larger and well-faceted rhombohedral crystallites with less agglomeration. As SEM pictures display in
Figure 2b the Rh-doped BaTiO
3 has the well-defined and homogeneously distributed spherical nanoparticles (~50 nm).
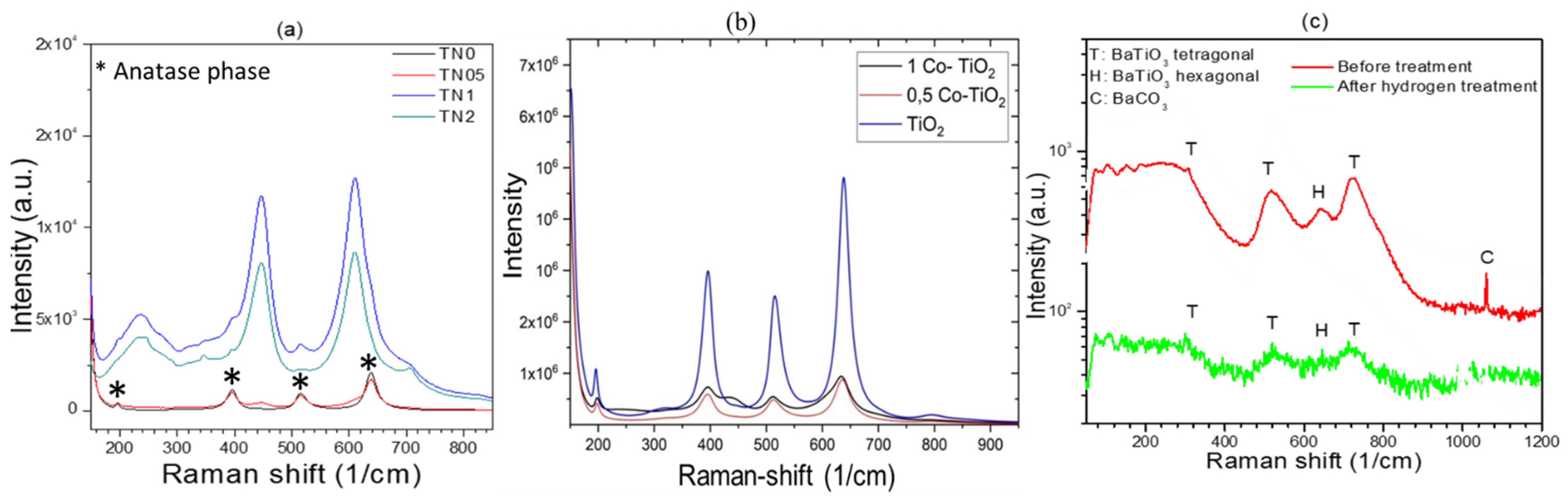
The Raman spectra of the samples were obtained between the wavenumbers of 175–800 cm
−1 and the results are presented in
Figure 3. The results of Ni-doped TiO
2 Raman analysis (
Figure 3a) show that the main signals came from TiO
2. The samples TN0 and TN05 show very strong Raman signals, with peaks at 196(E
g), 396(B1
g), 517(A1
g) and 638(B1g) cm
−1 from the typical anatase TiO
2 phase [
16]. A weak peak at 447 cm
−1 (E
g) attributed to rutile is observed in TN05. Samples TN1 and TN2, which contain larger amounts of Ni, present the Raman signals corresponding to both the anatase and rutile (447(E
g), 612(A1
g) cm
−1) phase [
17]. In addition to the anatase and rutile phases, another set of Raman vibrations emerges in the TN1 and TN2 samples. The peaks at 244, 345 and 706 cm
−1 are assigned to a trace amount of ilmenite, NiTiO
3. In the
Figure 3b showing the results of Co-doped TiO
2, the Raman lines observed at 197, 390, 511, 637 cm
−1 can be assigned to E
g, B1
g, A1
g, or B1
g and E
g modes of anatase phase respectively. The spectra show that the peak intensities decrease drastically after doping, due certainly to the decrease of the amount of anatase and the formation of rutile as indicated by XRD. Moreover, the Raman spectra of 1Co-doped TiO
2 yielded a smaller shift towards lower wavelengths while new peaks (436 cm
−1) appeared indicating the presence of rutile polymorph, as mentioned in literature [
17]. Raman spectra of Rh-doped BaTiO
3 are displayed in
Figure 3c. It shows the peaks at 270, 308, 525 and 725 cm
−1 which are assigned respectively to the A1(TO2), E(TO2), A1(TO3), and A1(LO3) of barium titanate modes of the room temperature P4mm phase.
Based on our previous results on undoped, Al and Cr-doped TiO
2, 600 °C was chosen as the optimum sensing temperature for Ni and Co doped TiO
2 in this work [
12,
13].
The responses of the undoped TiO
2 and all Ni-doped TiO
2 towards 10,000 ppm H
2 in dry synthetic air at 600 °C are shown in
Figure 4a. The sensor responses are 42, 72, 70 and 62% for TN0, TN05, TN1 and TN2, respectively. It can be observed that the sensor response increases greatly as Ni-content increases up to 0.5 mol.% and then decreases slowly with further increase in the Ni-content to 2.0 mol.%. This implies that the sensor reaches its maximum response of 72% with 0.5 mol.% of Ni dopant. It can be assumed that this enhancement of gas sensor response may be due to the formation of a n-n junction between the anatase (E
g = 3.2 eV) and rutile (E
g = 3.0 eV) phases. As revealed by XRD results, the TN05 sample contains almost the same amount of anatase and rutile phases (which is not the case with the other samples in this work), and thus, the highest amount of n-n junctions are expected to be present in this sample. This kind of junction effect has also been reported for other n-n junction systems such as ZnO-SnO
2 [
18] and SnO
2-WO
3 [
19]. The achievement of a great selectivity towards the target gas is a key parameter and a very important characteristic. Therefore, the responses of the TN05 gas sensor towards a variety of interference gases including NO
2, CO, and NO at 600 °C in dry synthetic air were explored to evaluate its selectivity. As observed in
Figure 4b, the response of this sensor towards 600 ppm of H
2 (35%) is at least a factor of two higher than that towards 300 ppm of CO (12%), 300 ppm of NO
2 (11%) and 300 ppm of NO (7%). The sensor’s H
2-response was a factor of two greater compared to that for 300 ppm of all the tested interfering gases, yielding the highest value and indicating a relatively high selectivity potential of the sensor towards H
2.As
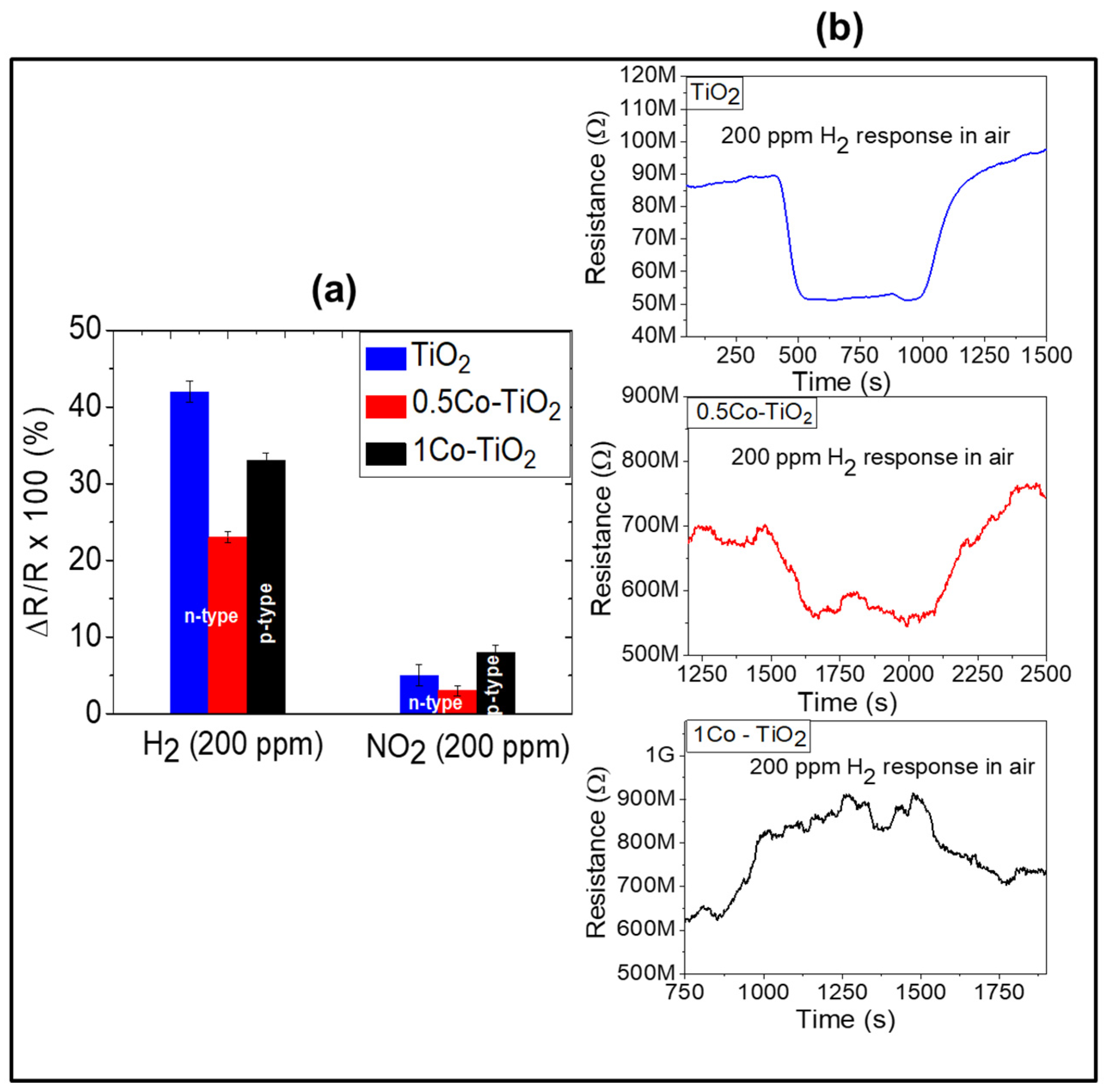
Figure 5a shows, the sensors yield higher response towards H
2 than NO
2. The H
2 sensor responses are 42, 23 and 33% for undoped, 0.5Co-doped TiO
2 and 1Co-doped TiO
2, respectively. The undoped sample shows the highest response toward H
2. The presence of Co-dopant seems to decrease the H
2-sensing performance of TiO
2 even though doping creates more oxygen vacancies in TiO
2. This behavior can be attributed to the increase of rutile polymorph content on doping with cobalt, as previously reported, rutile is the less active (in term of functional properties) polymorph of TiO
2 [
20]. On the other hand, the 1Co-doped TiO
2 which contains predominantly rutile polymorph showed a higher response toward H
2 than 0.5Co-doped TiO
2. This discrepancy can be explained by alteration of the conductivity from n-type to p-type. In fact, the dynamic response of the sensors toward H
2 given in
Figure 5b reveals that undoped and 0.5Co-doped TiO
2 exhibit n-type semi-conductivity (i.e., their electrical resistance decreases upon interaction with hydrogen) while the 1Co-doped TiO
2 yields p-type conductivity (its electrical resistance increases when reducing gas is introduced). The sensor responses measured toward the oxidizing gas NO
2 were 5, 3 and 8% for undoped, 0.5Co-doped TiO
2 and 1Co-doped TiO
2 respectively. In the case of NO
2 sensing, the 1Co-doped TiO
2 showed the highest response. This may be mainly due to the electronic alteration of TiO
2 from n to p-type semiconductor. Previous literature points out that this alteration can be utilized for the detection of oxidizing gas. Our current results confirm that the dominant factor for the gas sensing property of the Co-doped TiO
2 depend on the existing polymorphs as well as the nature of target gas (oxidizing or reducing). In the case of reducing gases, the type of polymorphs has more influence on the gas sensitivity than the type of electronic structure, while an opposite trend can be observed for oxidizing gases.
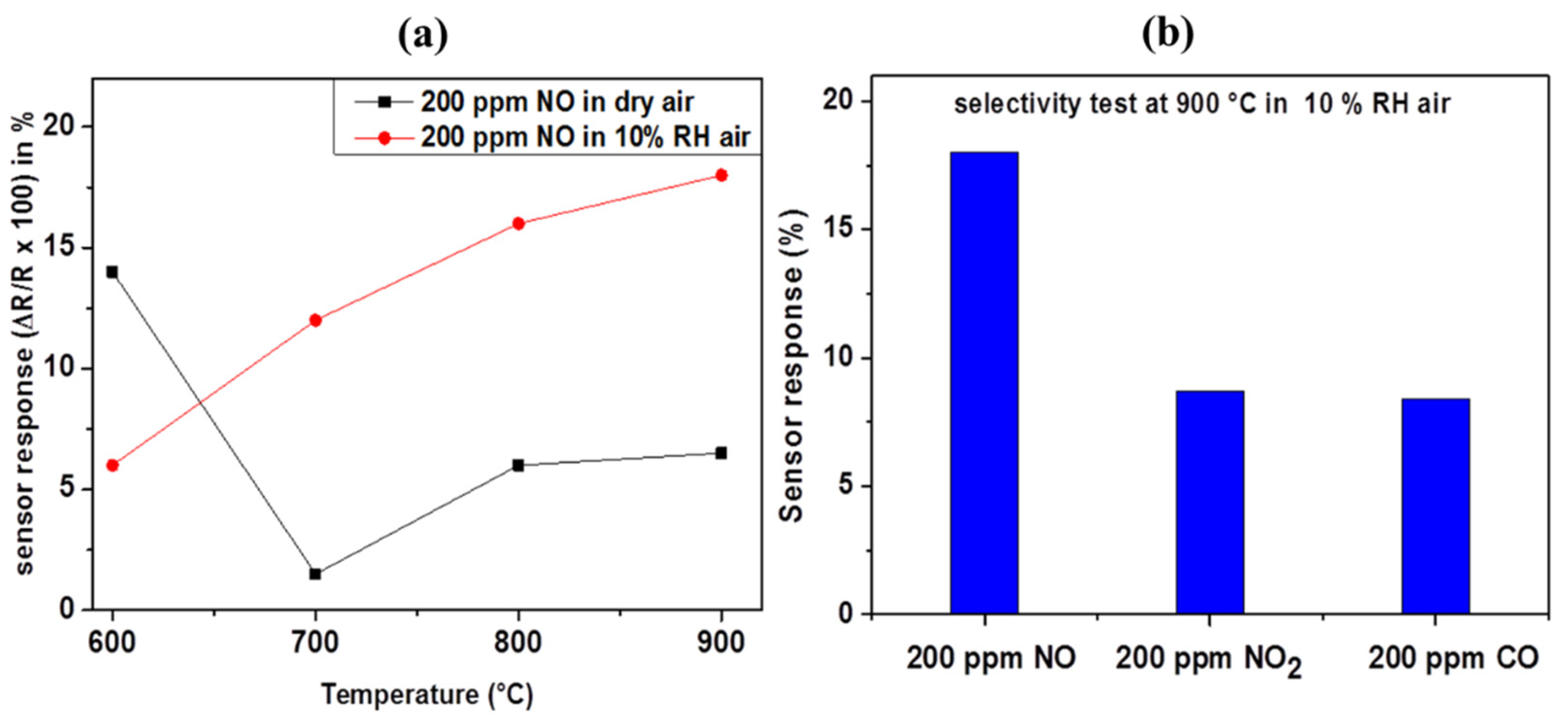
Figure 6a shows the sensor responses of the hydrogen treated Rh-doped BaTiO
3 towards 200 ppm of nitrogen oxide (NO) at a different operating temperature under dry and humid (10% of RH) synthetic air and the dynamic response at 700 and 900 °C, respectively. The sensor responses are 14, 2, 6, 7% in dry air, 6, 12, 16 and 18% in humid air at 600, 700, 800, and 900 °C, respectively. In dry air, the sensor response decreases from 14 to 7% in general with the increasing temperature, while in humid air, the sensor response increases from 6 to 18% as the temperature increases. The maximum sensor response is therefore obtained at 900 °C under humidity. This enhancement of sensing properties in the presence of humidity can be explained by the affinity between adsorbed hydroxyl group (generated after thermal decomposition of water) and the NO. In fact, at high temperatures, H
2O in water vapor decomposes, and hydroxyl is adsorbed on the sensing layer. As the temperature increases, more decomposition will occur, and more hydroxyl groups will be adsorbed on the surface, enhancing the NO sensor response. To the best of our knowledge, this is the first time that NO detection is reported at such a high temperature in the humid. Therefore, we have investigated intensely further gas sensing characteristics of this material under these extreme conditions (e.g., at 900 °C under humid air). Two other main products for fuel combustion are NO
2 and CO. Their presence in the exhaust gas stream at a high temperature can cause important hinderance for the NO gas sensing application. Our sensor’s selectivity toward NO against CO and NO
2 at 900 °C under humid air was investigated.
Figure 6b shows the different responses of the sensor to 200 ppm of NO, NO
2 and CO. The results indicate that at 900 °C, the response to 200 ppm of NO (18%) is higher than that of 200 ppm of NO
2 (8.7%) and 200 ppm of CO (8.4%). This implies that this sensor is at least twice as much sensitive to NO than NO
2 and CO. This good selectivity is ascribed to the catalytic effect of Rhodium-NPs on the oxidation of NO, which will promote and enhance the adsorption and the oxidation of NO preferentially. It is reported in the literature that Rhodium which is currently and often used in TWC, is a suitable catalyst for NO oxidation [
21].