Towards Low Temperature VOCs Chemoresistors: Graphene Oxide Versus Porphyrin-Based Materials †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hybrid Synthesis, Electrodes Preparation and Sensing Tests
2.2. Powders and Porphyrins Characterizations
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Ren, F.; Gao, L.; Yuan, Y.; Zhang, Y.; Alqrni, A.; Al-Dossary, O.M.; Xu, J. Enhanced BTEX gas-sensing performance of CuO/SnO2 composite. Sens. Actuators B Chem. 2016, 223, 914–920. [Google Scholar] [CrossRef]
- Pargoletti, E.; Hossain, U.H.; Di Bernardo, I.; Chen, H.; Tran-Phu, T.; Chiarello, G.L.; Lipton-Duffin, J.; Pifferi, V.; Tricoli, A.; Cappelletti, G. Engineering of SnO2–Graphene Oxide Nanoheterojunctions for Selective Room-Temperature Chemical Sensing and Optoelectronic Devices. ACS Appl. Mater. Interfaces 2020, 12, 39549–39560. [Google Scholar] [CrossRef] [PubMed]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath Sensors for Health Monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Pargoletti, E.; Hossain, U.H.; Di Bernardo, I.; Chen, H.; Tran-Phu, T.; Lipton-Duffin, J.; Cappelletti, G.; Tricoli, A. Room-temperature photodetectors and VOC sensors based on graphene oxide-ZnO nano-heterojunctions. Nanoscale 2019, 11, 22932–22945. [Google Scholar] [CrossRef] [PubMed]
- Pargoletti, E.; Verga, S.; Chiarello, G.L.; Longhi, M.; Cerrato, G.; Giordana, A.; Cappelletti, G. Exploring SnxTi1−xO2 Solid Solutions Grown onto Graphene Oxide (GO) as Selective Toluene Gas Sensors. Nanomaterials 2020, 10, 761. [Google Scholar] [CrossRef] [Green Version]
- Pargoletti, E.; Tricoli, A.; Pifferi, V.; Orsini, S.; Longhi, M.; Guglielmi, V.; Cerrato, G.; Falciola, L.; Derudi, M.; Cappelletti, G. An electrochemical outlook upon the gaseous ethanol sensing by graphene oxide-SnO2 hybrid materials. Appl. Surf. Sci. 2019, 483, 1081–1089. [Google Scholar] [CrossRef]
- Ekrami, M.; Magna, G.; Emam-djomeh, Z.; Yarmand, M.S.; Paolesse, R.; Di Natale, C. Porphyrin-Functionalized Zinc Oxide Nanostructures for Sensor Applications. Sensors 2018, 18, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carlo, G.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F.; Trifiletti, V.; Ruffo, R.; Abbotto, A.; Amat, A.; De Angelis, F.; Mussini, P.R. Tetraryl ZnII porphyrinates substituted at β-pyrrolic positions as sensitizers in dye-sensitized solar cells: A comparison with meso-substituted push-pull ZnII porphyrinates. Chem. Eur. J. 2013, 19, 10723–10740. [Google Scholar] [CrossRef] [PubMed]
- Orbelli Biroli, A.; Tessore, F.; Di Carlo, G.; Pizzotti, M.; Benazzi, E.; Gentile, F.; Berardi, S.; Bignozzi, C.A.; Argazzi, R.; Natali, M.; et al. Fluorinated ZnII porphyrins for dye-sensitized aqueous photoelectrosynthetic cells. ACS Appl. Mater. Interfaces 2019, 11, 32895–32908. [Google Scholar] [CrossRef] [PubMed]
- Berardi, S.; Caramori, S.; Benazzi, E.; Zabini, N.; Niorettini, A.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F.; Di Carlo, G. Electronic properties of electron-deficient Zn(II) porphyrins for HBr splitting. Appl. Sci. 2019, 9, 2739. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, G.; Orbelli Biroli, A.; Tessore, F.; Pizzotti, M.; Mussini, P.R.; Amat, A.; De Angelis, F.; Abbotto, A.; Trifiletti, V.; Ruffo, R. Physicochemical investigation of the panchromatic effect on β-substituted ZnII porphyrinates for DSScs: The role of the π bridge between a dithienylethylene unit and the porphyrinic ring. J. Phys. Chem. C 2014, 118, 7307–7320. [Google Scholar] [CrossRef]
- Americo, S.; Pargoletti, E.; Soave, R.; Cargnoni, F.; Trioni, M.I.; Chiarello, G.L.; Cerrato, G.; Cappelletti, G. Unveiling the acetone sensing mechanism by WO3 chemiresistors through a joint theory-experiment approach. Electrochim. Acta 2021, 371, 137611. [Google Scholar] [CrossRef]
- Anjali, K.; Jayaraj, L.K.N.; Ayyamperumal, C. Zinc-tetraphenylporphyrin grafted on functionalised mesoporous SBA-15: Synthesis and utilisation for nitroaldol condensation. J. Porous Mater. 2020, 27, 1191–1201. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Kang, S.; Mu, J. Preparation and enhanced visible light-driven catalytic activity of ZnO microrods sensitized by porphyrin heteroaggregate. Appl. Surf. Sci. 2010, 256, 6705–6709. [Google Scholar] [CrossRef]
- Chen, H.; Bo, R.; Shrestha, A.; Xin, B.; Nasiri, N.; Zhou, J.; Di Bernardo, I.; Dodd, A.; Saunders, M.; Lipton-Duffin, J.; et al. NiO–ZnO Nanoheterojunction Networks for Room-Temperature Volatile Organic Compounds Sensing. Adv. Opt. Mater. 2018, 6, 1800677. [Google Scholar] [CrossRef]
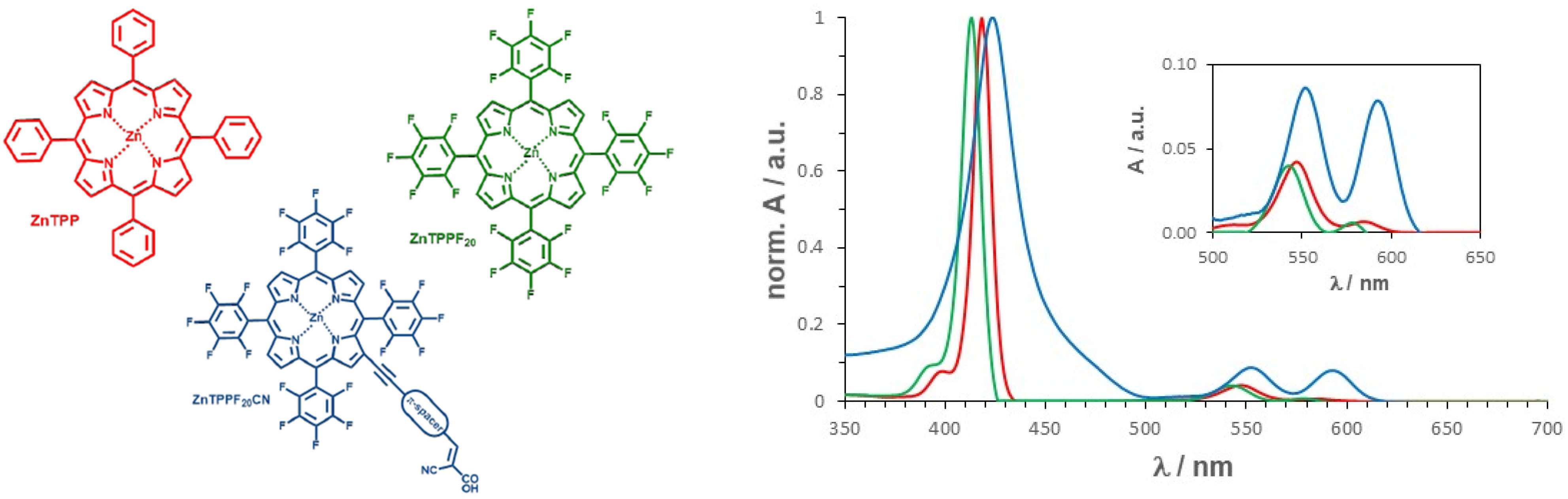
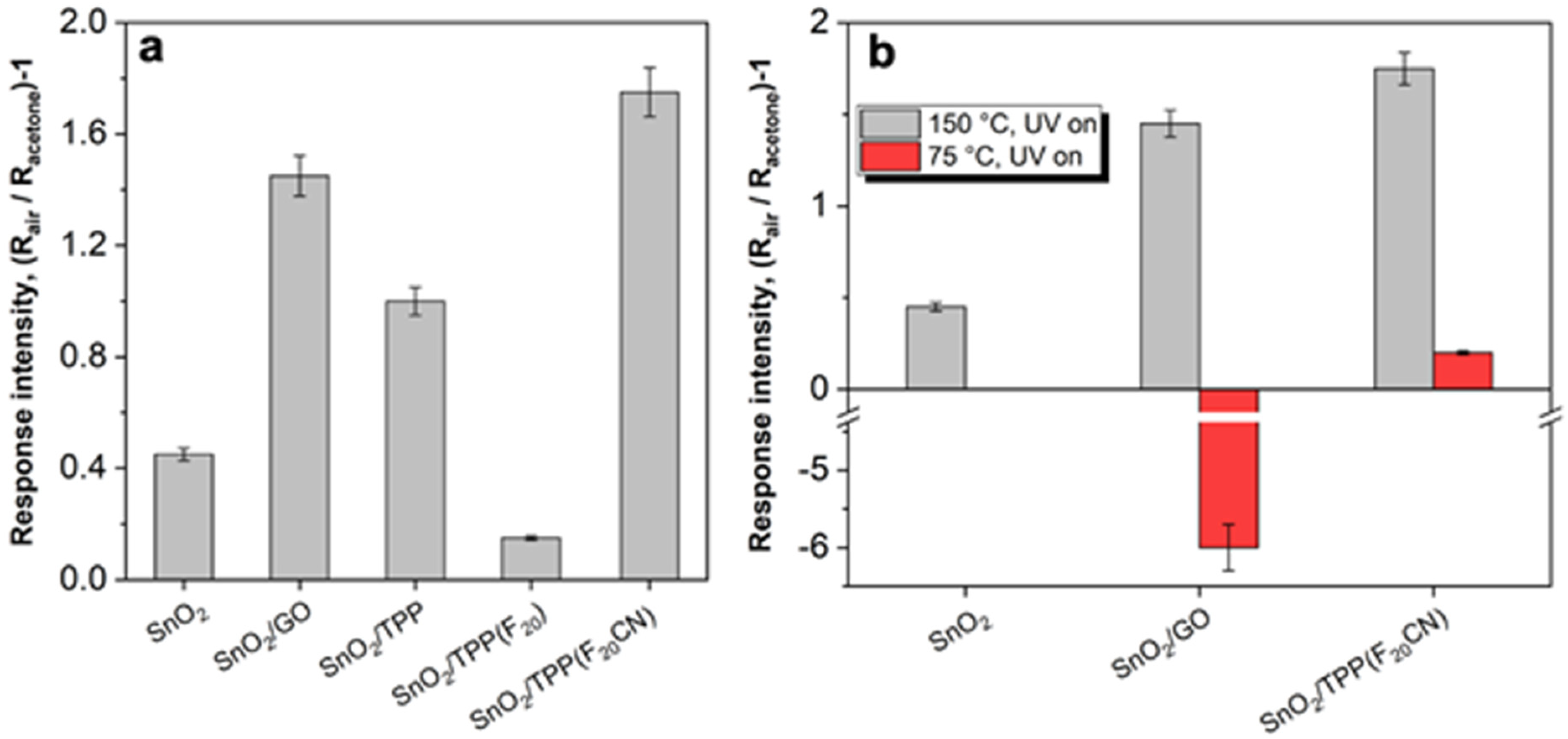
| Sample | SBET (m2 g−1) | Vtot. pores (cm3 g−1) | <dXRD> (nm) | Eg (eV) |
|---|---|---|---|---|
| GO | 30 | 0.020 | 11 | − |
| SnO2 | 67 | 0.210 | 15 | 3.6 |
| SnO2/GO | 55 | 0.133 | 8 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pargoletti, E.; Tessore, F.; Carlo, G.D.; Chiarello, G.L.; Cappelletti, G. Towards Low Temperature VOCs Chemoresistors: Graphene Oxide Versus Porphyrin-Based Materials. Chem. Proc. 2021, 5, 60. https://doi.org/10.3390/CSAC2021-10418
Pargoletti E, Tessore F, Carlo GD, Chiarello GL, Cappelletti G. Towards Low Temperature VOCs Chemoresistors: Graphene Oxide Versus Porphyrin-Based Materials. Chemistry Proceedings. 2021; 5(1):60. https://doi.org/10.3390/CSAC2021-10418
Chicago/Turabian StylePargoletti, Eleonora, Francesca Tessore, Gabriele Di Carlo, Gian Luca Chiarello, and Giuseppe Cappelletti. 2021. "Towards Low Temperature VOCs Chemoresistors: Graphene Oxide Versus Porphyrin-Based Materials" Chemistry Proceedings 5, no. 1: 60. https://doi.org/10.3390/CSAC2021-10418
APA StylePargoletti, E., Tessore, F., Carlo, G. D., Chiarello, G. L., & Cappelletti, G. (2021). Towards Low Temperature VOCs Chemoresistors: Graphene Oxide Versus Porphyrin-Based Materials. Chemistry Proceedings, 5(1), 60. https://doi.org/10.3390/CSAC2021-10418










