Optical Biosensor for the Detection of Hydrogen Peroxide in Milk †
Abstract
:1. Introduction
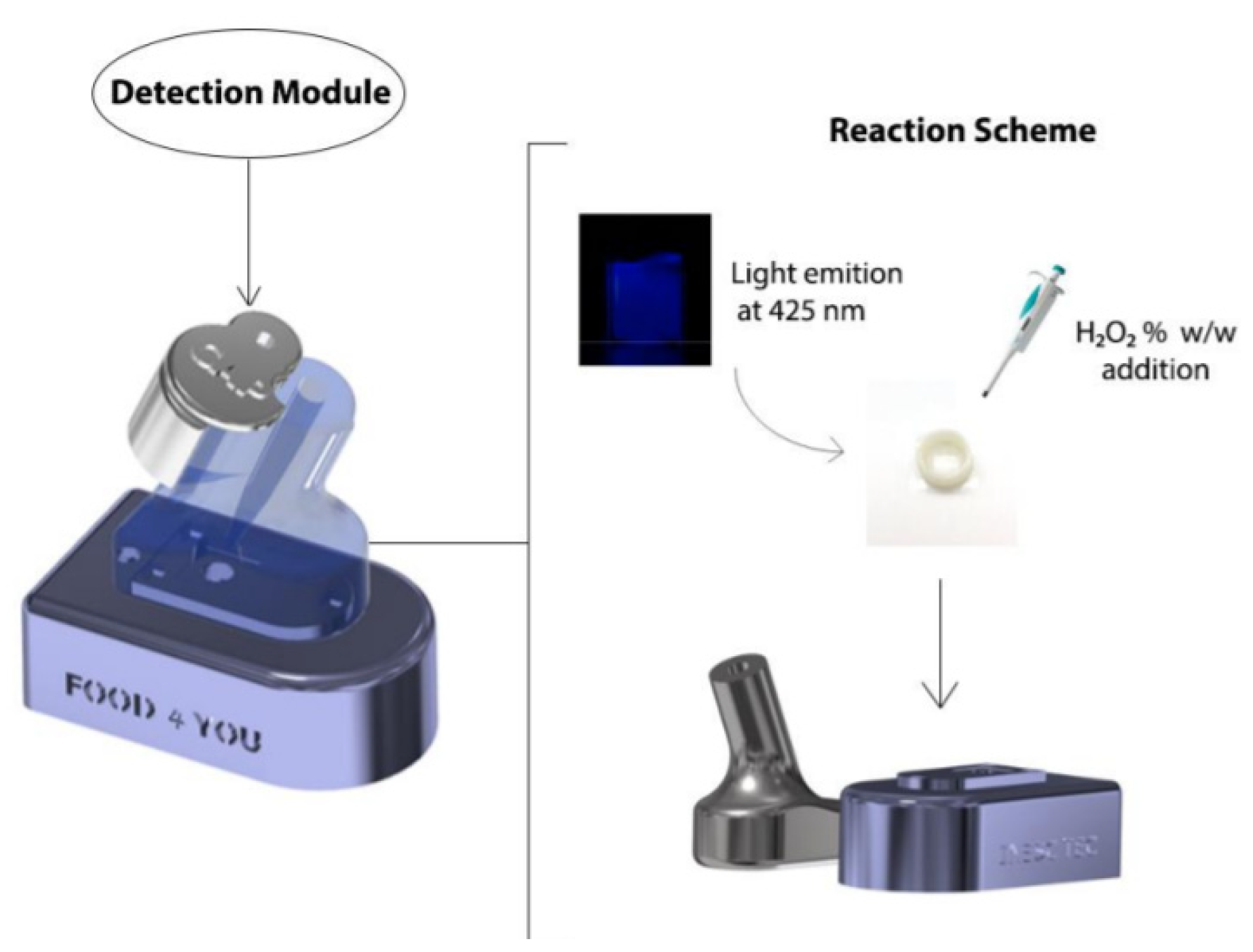
2. Materials and Methods
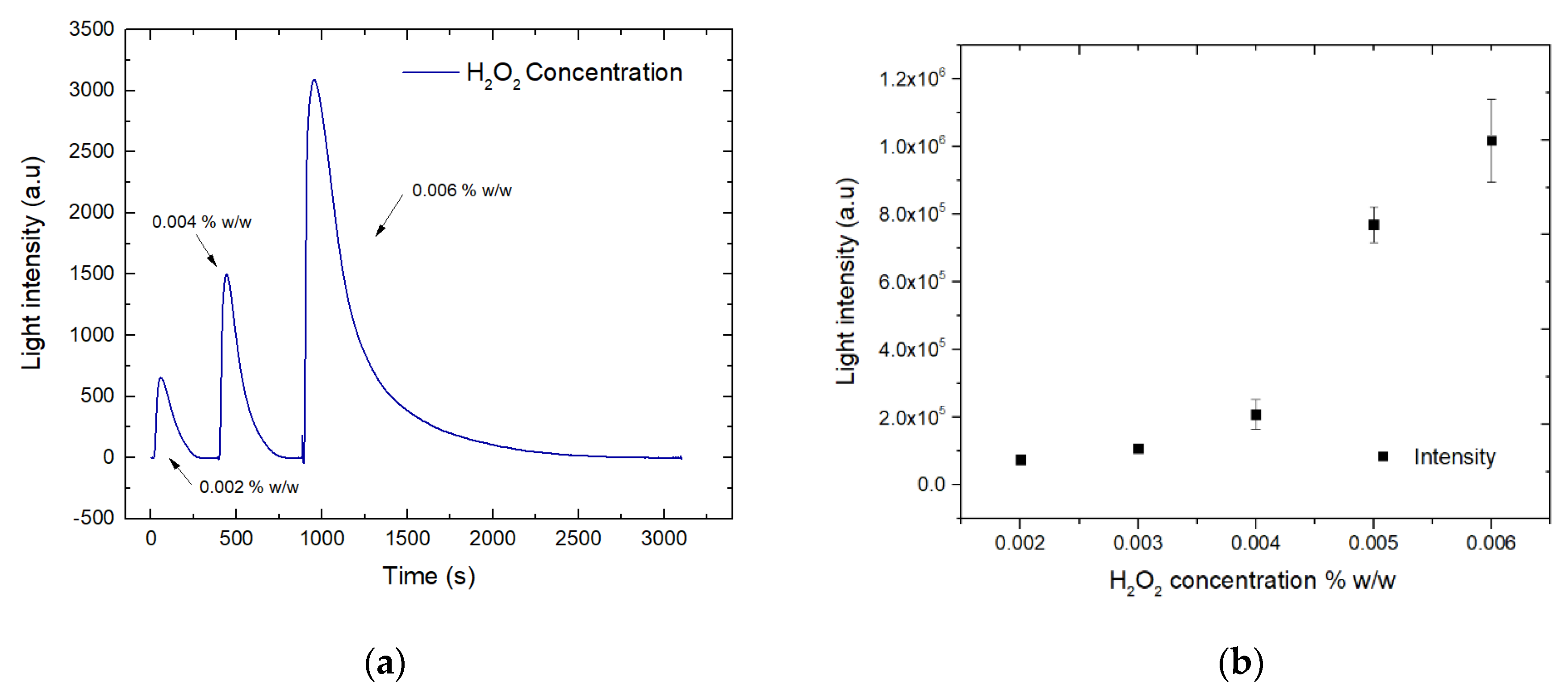
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Handford, C.E.; Campbell, K.; Elliott, C.T. Impacts of Milk Fraud on Food Safety and Nutrition with Special Emphasis on Developing Countries. Compr. Rev. Food Sci. Food Saf. 2016, 15, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, L.S.; Rossini, E.L.; Pezza, L.; Pezza, H.R. Bioactive Paper Platform for Detection of Hydrogen Peroxide in Milk. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 227, 117774. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.A.; Sasaki, M.K.; Marinho, O.R.; Freitas, T.A.; Faria, R.C.; Reis, B.F.; Rocha, F.R.P. Spot Test for Fast Determination of Hydrogen Peroxide as a Milk Adulterant by Smartphone-Based Digital Image Colorimetry. Microchem. J. 2020, 157, 105042. [Google Scholar] [CrossRef]
- Robinson, B.R.; D’Amico, D.J. Hydrogen Peroxide Treatments for the Control of Listeria Monocytogenes on High-Moisture Soft Cheese. Int. Dairy J. 2021, 114, 104931. [Google Scholar] [CrossRef]
- Omanovic-Miklicanin, E.; Valzacchi, S. Development of New Chemiluminescence Biosensors for Determination of Biogenic Amines in Meat. Food Chem. 2017, 235, 98–103. [Google Scholar] [CrossRef] [PubMed]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 6 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, H.; Matias, A.; Jorge, P.; Saraiva, C.; Mendes, J.; Araújo, J.; Dias, B.; Santos, P.; Almeida, J.M.M.M.; Coelho, L.C.C. Optical Biosensor for the Detection of Hydrogen Peroxide in Milk. Chem. Proc. 2021, 5, 55. https://doi.org/10.3390/CSAC2021-10466
Vasconcelos H, Matias A, Jorge P, Saraiva C, Mendes J, Araújo J, Dias B, Santos P, Almeida JMMM, Coelho LCC. Optical Biosensor for the Detection of Hydrogen Peroxide in Milk. Chemistry Proceedings. 2021; 5(1):55. https://doi.org/10.3390/CSAC2021-10466
Chicago/Turabian StyleVasconcelos, Helena, Ana Matias, Pedro Jorge, Cristina Saraiva, João Mendes, João Araújo, Bernardo Dias, Paulo Santos, José M. M. M. Almeida, and Luís C. C. Coelho. 2021. "Optical Biosensor for the Detection of Hydrogen Peroxide in Milk" Chemistry Proceedings 5, no. 1: 55. https://doi.org/10.3390/CSAC2021-10466
APA StyleVasconcelos, H., Matias, A., Jorge, P., Saraiva, C., Mendes, J., Araújo, J., Dias, B., Santos, P., Almeida, J. M. M. M., & Coelho, L. C. C. (2021). Optical Biosensor for the Detection of Hydrogen Peroxide in Milk. Chemistry Proceedings, 5(1), 55. https://doi.org/10.3390/CSAC2021-10466










