1. Introduction
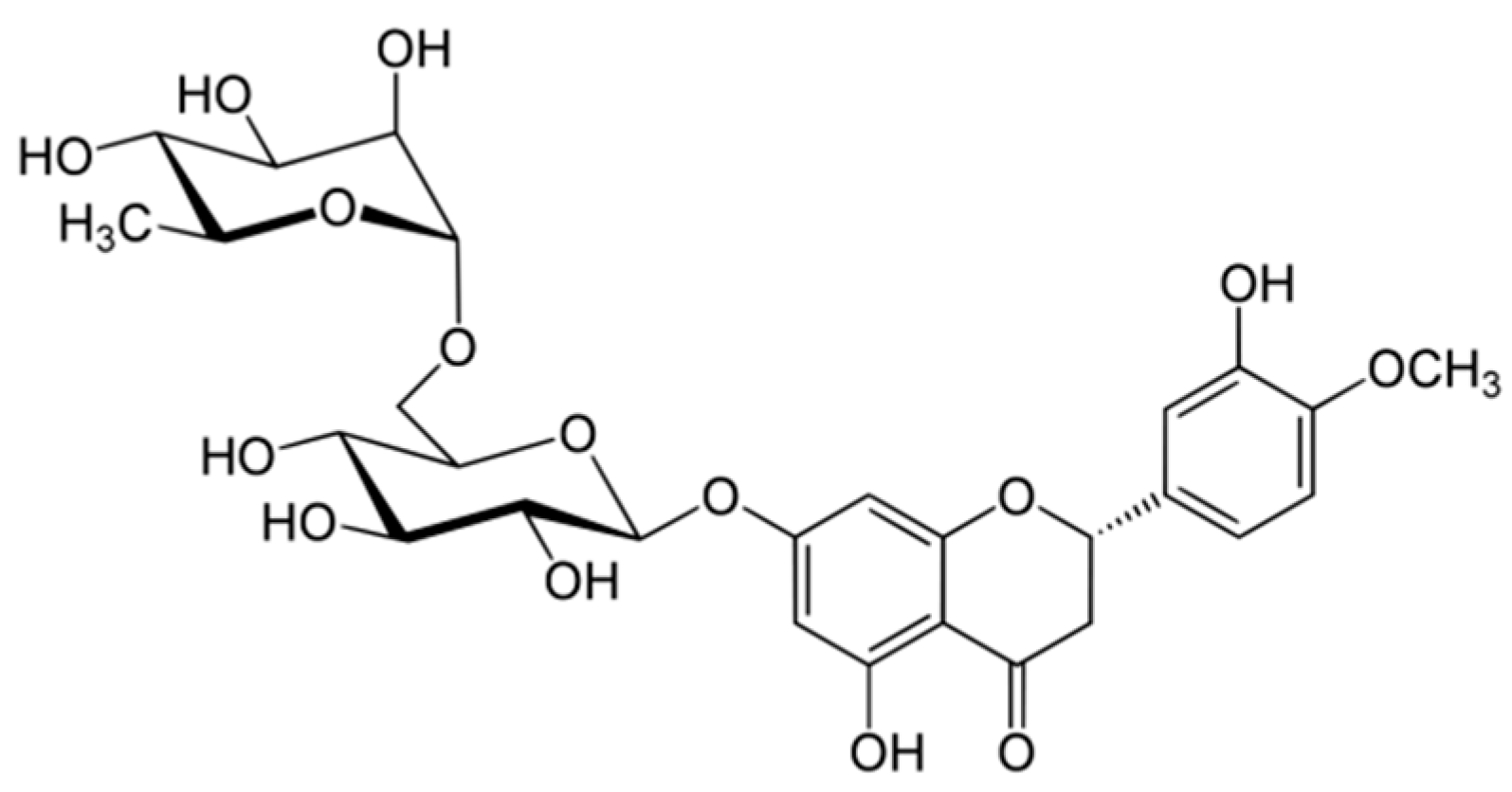
Hesperidin (
Figure 1) is the major flavonoid of
Citrus L. fruits [
1], with a wide spectrum of biological activity that results in its application in medicine [
2]. However, it can show a pro-oxidant effect in high concentrations that is typical for phenolic antioxidants [
3]. Therefore, simple, sensitive, and selective methods for hesperidin determination are required.
Electrochemical sensors can be successfully used for this purpose due to the ability of hesperidin to be oxidized at the electrode surface. The advantages of electrochemical methods, such as their simplicity, portability, and cost-efficiency in combination with reliability, sensitivity, and sufficient selectivity make them an attractive tool for practical applications. Nevertheless, hesperidin is almost disregarded as an analyte in electroanalysis compared to other natural flavonoids. Thus, the development of electrochemical sensors for hesperidin quantification is of interest from scientific and practical points of view.
The hanging drop mercury electrode has been used for hesperidin quantification [
4,
5]. Significant interference effects from a wide range of inorganic and organic compounds of different classes, as well as the toxicity of mercury, make these methods inapplicable in laboratory practice. Boron-doped diamond [
6] and pencil graphite [
7] electrodes allow the limitations mentioned above to be partially overcome, although the selectivity of the hesperidin response is still insufficient. At the present time, chemically modified electrodes are applied in hesperidin analysis [
8,
9,
10,
11,
12,
13,
14,
15] since they provide higher sensitivity and selectivity of response. The analytical characteristics of hesperidin reported for the existing electrochemical sensors are presented in
Table 1. Nevertheless, the selectivity of the sensor’s response is often not considered or is insufficient, and there is limited applicability to real samples. The narrow linear dynamic range of the sensor response in some cases also complicates the analysis of real samples. Therefore, further development of electrochemical sensors for hesperidin that are free of these limitations is required.
Electrochemically inert metal oxide nanoparticles (CeO
2, TiO
2, ZnO, SnO
2, Fe
3O
4, etc.) are prospective nanomaterials widely used for voltammetric sensor creation [
16,
17,
18,
19,
20,
21,
22]. The combination of metal oxide nanoparticles with surfactants as dispersive agents has led to significant improvement in the voltammetric response of phenolic antioxidants [
16,
17,
18,
19], caused by stabilization of the nanoparticle dispersions on the one hand and, on the other hand, preconcentration of the analyte at the sensitive layer of the sensor surface via electrostatic or hydrophobic interactions. Another important aspect to be taken into account is the increase in the sensor conductivity due to the presence of surfactants since the metal oxide nanoparticles mentioned above are semiconductors. This type of electrode surface modifier has been successfully applied in the electroanalysis of natural phenolic antioxidants, particularly eugenol [
16], thymol [
17], quercetin and rutin [
18], vanillin [
19], and gallic acid [
20,
21], and in the simultaneous detection of synapic and syringic acids and rutin [
22]. The sensors show high sensitivity and selectivity for the response and are easy to prepare, which is an advantage over other modified electrodes. No electrochemical sensors based on metal oxide nanoparticles for hesperidin determination have been reported to date, although it is of practical interest.
The current study was focused on the creation and application of a novel voltammetric sensor for hesperidin based on a glassy carbon electrode (GCE) modified with tin(IV) oxide nanoparticles and surfactants. Attention was paid to the evaluation of the effect of the surfactant’s nature and concentration on the hesperidin voltammetric response. The electrodes under investigation were characterized by scanning electron microscopy (SEM) and electrochemical methods. The analytical aspects of hesperidin detection are discussed.
2. Materials and Methods
Hesperidin (94% purity) from Sigma (Steinheim, Germany) was used as a standard. A 0.40 mM stock solution was prepared in methanol (cp grade). Naringin (95% purity), 99% ascorbic and 98% caffeic acids, 95% quercetin trihydrate, and 85% morin hydrate from Sigma (Steinheim, Germany), 95% chlorogenic acid from Aldrich (Steinheim, Germany), and 97% rutin trihydrate from Alfa Aesar (Heysham, UK) were used in the interference test. For these, 10 mM stock solutions in methanol were prepared in 5.0 mL flasks. Less-concentrated solutions were obtained by exact dilution.
Tin(IV) oxide nanoparticles (D < 100 nm) were purchased from Aldrich (Steinheim, Germany). Their 1 mg mL−1 dispersions in water and the surfactants were prepared by sonication for 10 min in a WiseClean WUC-A03H ultrasonic bath (DAIHAN Scientific Co., Ltd., Wonju-si, Korea). Cetylpyridinium bromide (CPB) (98% purity), 97% N-lauroylsarcosine sodium salt (LSS), and Triton X-100 from Aldrich (Steinheim, Germany), 99% cetyltrimethylammonium bromide (CTAB) and Brij® 35 from Acros Organics (Geel, Belgium), sodium dodecylsulfate (SDS) (Ph. Eur.) from Panreac (Barcelona, Spain), and cetyltriphenylphosphonium bromide (CTPPB) synthesized in the Department of Organoelement Compounds Chemistry of Kazan Federal University were used as dispersive agents. Their 1.0 mM solutions were prepared in distilled water.
Other reagents were chemical grade purity. Double-distilled water was used for the measurements. The experiments were carried out at laboratory temperature (25 ± 2 °C).
Voltammetric measurements were carried out on an Autolab PGSTAT12 potentiostat/galvanostat (Eco Chemie B.V., Utrecht, The Netherlands) with GPES software, version 4.9.005. Electrochemical impedance spectroscopy was performed on an Autolab PGSTAT302N potentiostat/galvanostat with a FRA32M module (Eco Chemie B.V., Utrecht, Netherlands) and NOVA 1.10.1.9 software. A 10 mL glassy electrochemical cell with working GCE with a 7.07 mm2 geometric surface area (BASi® Inc., West Lafayette, IN, USA) or a modified electrode, a silver–silver-chloride-saturated KCl reference electrode, and a platinum wire as the counter electrode was used.
An Expert-001 pH meter (Econix-Expert Ltd., Moscow, Russian Federation) equipped with the glassy electrode was used for pH measurements.
SEM was carried out on a MerlinTM high-resolution field-emission scanning electron microscope (Carl Zeiss, Oberkochen, Germany) at an accelerating voltage of 5 kV and an emission current of 300 pA.
3. Results and Discussion
3.1. Voltammetric Characteristics of Hesperidin on Modified Electrodes
The voltammetric behavior of hesperidin on bare GCE and the modified electrodes was studied in 0.1 M phosphate buffer at pH 7.0. Hesperidin is irreversibly oxidized in two steps. The second step is less pronounced. Therefore, the first oxidation peak was used (
Table 2). Modification of the electrode surface with tin(IV) oxide nanoparticles provided an insignificant increase in the hesperidin oxidation currents. Furthermore, these values were still insufficient for sensitive hesperidin quantification. The use of surfactants provided stabilization of the nanoparticle dispersions and preconcentration of the hesperidin on the electrode surface via hydrophobic interaction, leading to an increase in the oxidation currents for all the surfactants under investigation. The effect of surfactant concentration in the range of 10–500 µM on the hesperidin response was evaluated. The oxidation potentials were cathodically shifted. The oxidation currents were statistically significantly increased. Higher oxidation currents were obtained in the case of cationic surfactants. The best hesperidin response was registered on the sensor based on tin(IV) oxide nanoparticles dispersed in 500 µM cetylpyridinium bromide. This agrees well with literature data for a cerium-dioxide-nanoparticles-based sensor for eugenol [
16] and a tin-dioxide-nanoparticles-based sensor for vanillin [
19].
3.2. Electrodes Characterization via SEM and Electrochemical Methods
SEM shows the presence of spherical and rhomboid structures and their aggregates of size 30–200 nm for SnO
2-H
2O/GCE, in contrast to the relatively smooth surface of GCE (
Figure 2a,b). The application of CPB as dispersive agent provides more uniform coverage consisting of spherical particles of size 20–40 nm forming a porous surface leading to an increase in the electrode surface area (
Figure 2c).
The electroactive surface area of the modified electrode is significantly increased compared with bare GCE (34.7 ± 0.3 mm2 for SnO2-CPB/GCE and 8.9 ± 0.3 mm2 for GCE), as confirmed by cyclic voltammetry (for SnO2-CPB/GCE) and chronoamperometry (for GCE and SnO2-H2O/GCE) using [Fe(CN)6]4− ions as a standard. These data explain the increase in hesperidin oxidation currents on the modified electrode. Electrochemical impedance spectroscopy was performed in the presence of [Fe(CN)6]4−/3− as a redox probe at 0.23 V. Fitting of the impedance spectra using the Randles equivalent circuit showed 554-fold less charge transfer resistance for the modified electrode in comparison to GCE, indicating a dramatic increase in the electron transfer rate. The constant phase element value for SnO2-CPB/GCE was 4.7-fold higher than for the GCE due to the porous structure of the modified electrode and the increase in the surface total charge due to the presence of positively charged CPB. Thus, the developed sensor can be considered as a candidate for analytical applications.
3.3. Analytical Characterization of the Sensor
Hesperidin quantification using the developed sensor was performed in adsorptive differential pulse mode since surface-controlled electro-oxidation has been proved. The highest oxidation currents for hesperidin were obtained in 0.1 M phosphate buffer at pH 7.0. The variation of preconcentration time at the open circuit potential showed the highest oxidation currents for 120 s of accumulation. The evaluation of the effect of the pulse parameters showed that the best response was registered at a pulse amplitude of 100 mV and a pulse time of 50 ms.
The sensor gave a linear response to hesperidin in the ranges of 0.10–10 and 10–75 µM (
Figure 3) with a detection limit of 77 nM. The calibration-plot parameters are presented in
Table 3.
The analytical characteristics obtained were significantly better [
10,
15] or comparable to other sensors based on the modified electrodes [
12,
13,
14]. However, the sensor developed is simpler, relatively cheaper, and less tedious to prepare. The accuracy of the hesperidin determination was tested for the model solutions using the added–found method (
Table 4). The recovery of 98.4–100% confirmed the high accuracy of the developed sensor. The relative standard deviation was less than 3.5%, indicating the absence of random errors of quantification and the high reproducibility of the sensor response since surface renewal was performed after each measurement.
The sensor selectivity in the presence of a 1000-fold excess of inorganic ions (K+, Mg2+, Ca2+, NO3−, Cl−, and SO42−), glucose, rhamnose, and sucrose, as well as a 1000-fold excess of ascorbic acid was demonstrated. Another important advantage was the high selectivity to hesperidin in the presence of other flavonoids and phenolic acids. A 10-fold excess of naringin, quercetin, rutin, morin, and caffeic and chlorogenic acids, despite the fact that they are electroactive, did not result in an interference effect in the hesperidin response.
3.4. Application to Real Samples
The sensor’s applicability to the analysis of real samples was successfully tested on orange juices. The following sample preparation was applied before the measurements: 6 mL of juice was mixed with 6 mL of methanol, sonicated for 15 min, and filtered through 0.45 µm pore size nylon membrane filters [
23].
There is a well-defined oxidation peak of hesperidin on the differential pulse voltammograms of orange juices (commercial and fresh) that was confirmed by the standard addition method (
Figure 4). Recovery values of 99–100% indicated the absence of matrix effects in the determination.
The results of the hesperidin quantification in orange juices using the developed sensor are presented in
Figure 5. Validation with independent ultra-HPLC with mass-spectrometric detection results was performed (
Figure 5). The relative standard deviation for both methods did not exceed 2%, proving the absence of random errors. The
t-test values (0.290–1.08) were less than the critical value of 2.45, confirming the absence of systematic errors in the determination. Similarly, the
F-test results (1.17–2.57) were less than critical value of 6.59, indicating the uniform precision of the methods used.
4. Conclusions
A sensor based on tin(IV) oxide nanoparticles and CPB provide an improvement in the voltammetric and analytical characteristics of hesperidin. The surfactant provides stabilization of the nanomaterial dispersion and the accumulation of analyte on the sensor surface. The novel voltammetric sensor is highly sensitive, selective, and reliable and can be recommended for the preliminary screening of citrus juices as an alternative to chromatography.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z. and E.Y.; investigation, E.Y.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, G.Z. and E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Irina Galkina (Department of High Molecular and Organoelement Compounds, Kazan Federal University) for the synthesis and granting of CTPPB, Rustam Davletshin (Department of High Molecular and Organoelement Compounds, Kazan Federal University) for the chromatographic measurements, and Aleksei Rogov (Laboratory of Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the SEM measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Obendorf, D.; Reichart, E. Determination of hesperidin by catholic stripping voltammetry in orange juice and helopyrin, a phytopharmaceutical preparation. Electroanalysis 1995, 7, 1075–1081. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, M.S.; Kotb, M. Square-wave cathodic adsorptive stripping voltammetric determination of 3-hydroxyflavone, morin and hesperidin in bulk form and biological fluids in absence and presence of Cu(II). J. Braz. Chem. Soc. 2011, 22, 2056–2064. [Google Scholar] [CrossRef] [Green Version]
- Yiğit, A.; Yardım, Y.; Şentürk, Z. Square-wave adsorptive stripping voltammetric determination of hesperidin using a boron-doped diamond electrode. J. Anal. Chem. 2020, 75, 653–661. [Google Scholar] [CrossRef]
- David, I.G.; Numan, N.; Buleandră, M.; Popa, D.-E.; Lițescu, S.C.; Riga, S.; Ciobanu, A.M. Rapid voltammetric screening method for the assessment of bioflavonoid content using the disposable bare pencil graphite electrode. Chemosensors 2021, 9, 323. [Google Scholar] [CrossRef]
- Sims, M.J.; Li, Q.; Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using multiwalled carbon nanotube modified electrodes for the adsorptive striping voltammetric determination of hesperidin. Electrochim. Acta 2009, 54, 5030–5034. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Wang, Q.; Zou, L.; Ye, B. The novel voltammetric method for determination of hesperetin based on a sensitive electrochemical sensor. Talanta 2016, 150, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, F.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical determination of hesperidin using mesoporous SiO2 modified electrode. Microchim. Acta 2009, 167, 35–39. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, H.; Lu, W.; Guo, M. Highly sensitive detection of hesperidin using AuNPs/rGO modified glassy carbon electrode. Analyst 2018, 143, 297–303. [Google Scholar] [CrossRef]
- Sun, B.; Hou, X.; Li, D.; Gou, Y.; Hu, F.; Li, W.; Shi, X. Electrochemical sensing and high selective detection of hesperidin with molecularly imprinted polymer based on ultrafine activated carbon. J. Electrochem. Soc. 2019, 166, B1644–B1652. [Google Scholar] [CrossRef]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Bhakta, A.K.; Mekhalif, Z. Nano-graphene-platelet/Brilliant-green composite coated carbon paste electrode interface for electrocatalytic oxidation of flavanone hesperidin. Microchem. J. 2021, 160 B, 105768. [Google Scholar] [CrossRef]
- Tığ, G.A.; Bolat, E.Ö.; Zeybek, B.; Pekyardımcı, Ş. Hesperidin-dsDNA interaction based on electrochemically reduced graphene oxide and poly-(2,6-pyridinedicarboxylic acid) modified glassy carbon electrode. Hacet. J. Biol. Chem. 2016, 44, 487–497. [Google Scholar]
- Ziyatdinova, G.; Ziganshina, E.; Romashkina, S.; Budnikov, H. Highly sensitive amperometric sensor for eugenol quantification based on CeO2 nanoparticles and surfactants. Electroanalysis 2017, 29, 1197–1204. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Nguyen Cong, P.; Budnikov, H. Voltammetric determination of thymol in oregano using CeO2-modified electrode in Brij® 35 micellar medium. Food Anal. Meth. 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Zakharova, S.P.; Ziganshina, E.R.; Budnikov, H.C. Voltammetric determination of flavonoids in medicinal plant materials using electrodes modified by cerium dioxide nanoparticles and surfactants. J. Anal. Chem. 2019, 74, 816–824. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H.C. An amperometric sensor based on tin dioxide and cetylpyridinium bromide nanoparticles for the determination of vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Chikere, C.; Faisal, N.H.; Lin, P.K.T.; Fernandez, C. Zinc oxide nanoparticles modified-carbon paste electrode used for the electrochemical determination of gallic acid. J. Phys. Conf. Ser. 2019, 1310, 012008. [Google Scholar] [CrossRef] [Green Version]
- Tashkhourian, J.; Nami Ana, S.F.; Hashemnia, S.; Hormozi-Nezhad, M.R. Construction of a modified carbon paste electrode based on TiO2 nanoparticles for the determination of gallic acid. J. Solid State Electrochem. 2013, 17, 157–165. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical sensor for determination of various phenolic compounds in wine samples using Fe3O4 nanoparticles modified carbon paste electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Q.; Cong, P.; Zhu, Z. Simultaneous determination of flavonoids in different citrus fruit juices and beverages by high-performance liquid chromatography and analysis of their chromatographic profiles by chemometrics. Anal. Methods 2012, 4, 3748–3753. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).