Abstract
This paper presents a system for the measurement of chlorides in drinking and wastewater, based on an electrochemical process using a selective electrode as a transducer, which was developed by this group. The measurement for the concentration is carried out by introducing the implemented electrode (considered as reference) in the sample that will be analyzed; then a current is passed producing a potential difference in the system. Different aqueous solutions of sodium chloride (NaCl) were used, ranging between 35 and 3546 µg of chloride ions (Cl−). As a data acquisition and monitoring system for the analysis, an ATmega 328P microcontroller was used as the main capture element for subsequent interpretation through graphics. The experimental results show that it was possible to detect a potential difference in the electrochemical measurement system that corresponded to 35 µg of chloride ions (Cl−), making clear the detection process and the selectivity of chloride ions. It is important to mention that with this measurement system and the applied methodology, results are obtained in real time using a small sample volume and without generate ng extra liquid waste, compared to the application of the traditional analytical titrimetric method. Finally, this chloride measurement system is inexpensive and can be used in drinking and wastewater measurements.
1. Introduction
Measurement by electrometric methods continues to be useful in the analytical identification processes of ion-selective electrodes (ISEs) of potentiometric sensors [1,2] because only small dimensions are involved and measurements can be made requiring little sample volume with respect to the activity of the ions in solution [3,4]. With the increasing demand for environmentally friendly technologies and in situ measurements, these designs facilitate quick readings and detection of low concentrations [1,2], as observed when obtaining measurements of concentrations of 35 µg/L in NaCl solutions [5].
Due to the diverse range of applications and reduced operating times, there is a need for measurement electrodes that are selective and able to detect a specific ion of a species that interacts with it using potential difference [4,6]. Several electrodes have been developed for selective ion determination which involve use of an ionophore to generate exchange in a solution enabling determination of the influence of the exchange ion percentage [7,8,9,10].
In this study, a chloride measurement system for drinking and wastewater is proposed based on an electrochemical process using a selective electrode as a transducer which was developed by this group. The ionophore that is proposed is an AgNO3 solution with an extra modification that relates to the use of a metal that interacts with a natural organic membrane, modifying the attraction of the ion, which enhances the selectivity to the desired ion (Cl−) [3,11], in addition to the copper reference electrode [9].
2. Materials and Methods
The experimental arrangement of the electrochemical measurement system, shown in Figure 1, is made up of two essential blocks: the electrochemical half-cell and the data acquisition system.
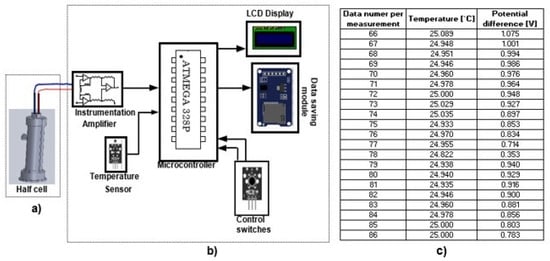
Figure 1.
Experimental arrangement of the electrochemical measurement system. (a) Electrochemical half-cell; (b) Data acquisition system; (c) Temperature and potential difference data obtained directly from the measuring device.
For the half-cell (Figure 1a), a 3D design was produced and printed to ensure the electrode separation was constant and to reduce movements in the experimental arrangement. The electrochemical half-cell was constructed with an active noble metal electrode (X) and a copper reference electrode, which are submerged in different solutions, such as AgNO3 and NaCl, respectively, according to Creus (2011) [12] and previous research by Toriz, Loredo and Marcial [13].
The measurement of chloride ions (Cl−) was achieved through reaction in the half-cell taking as analytes NaCl solutions at different concentrations, in which an ionic migration was generated through an organic (natural) membrane. The organic membrane and the inert metallic material (X) allow the potential difference to remain active during the measurement.
The coefficients of total ionic activity are related to the individual coefficients of ionic activity, detecting free energy in the system that connects the thermodynamics of the reaction with the values of the electromotive force (emf) [10] and that can only be applied in reversible reactions. This can be represented by Equation (1).
where Ɛ° is the potential difference, ΔF° is the total free energy of the system, n is the number of moles used in the reaction, and Ꞙ = Farad (free energy value = 96,500 Joules: approximate value = 23,052 cal).
The electromotive force (emf) depends on the concentration of the cation according to the Nernst equation [14]. According to the literature, the relationship between the signs ∆F° and Ɛ° correspond directly to a spontaneous reaction (Maron and Prutton) [9].
Referring to a standard emf when the activity is a unit, the fundamental equation can be related to the thermodynamic equation and the emf values. The values of Ɛ° can be tabulated for each reaction of a half-cell and added algebraically to obtain the value of Ɛ°.
To calculate the total free energy of the system, the relationship with thermodynamics is considered, calculating the emf from enthalpy and entropy tables for the reference half-cell [9,10], where T = 298 K, applying the relationship described in Equation (2):
The calculation of the total ΔF° corresponds to the sum of the cathodes of the system; the reaction of each cell is stated as in Equation (3):
Substituting the values for the half-cell reaction of the system (total ΔF°), Equation (4) is obtained:
Solving Equation (4), the results presented in Equation (5) are obtained:
Considering n = 1 and substituting the values in the equation, the standard potential of the system will be defined by Equation (6):
The value obtained from Equation (6) represents the emf of the ionic activity with respect to the constant concentration of the ionic activity of the cell. It provides the voltage difference with respect to the element. This theoretical value was obtained experimentally using the measurements presented in Figure 1c. The relationship of this value and the concentration was used to establish the scale.
The data acquisition system is mainly comprised of an ATMEGA328P microcontroller that multiplexes two analog channels to store the temperature data and the potential difference of the half-cell in the microSD memory module for subsequent graphic analysis. The data from the potential difference of the electrodes are acquired through an instrumentation amplifier with unity gain to reduce the effects of electrical noise that may exist in the system (Figure 1b). The system also has an LCD module that prints the values of the analog channels sampled in the microcontroller (temperature and potential difference of the half-cell) every 5 s to verify changes in real time. When acquiring measurements with this system, the data id was saved in an Excel file where the first column shows the temperature and the second column the potential difference (Figure 1c).
3. Results and Discussion
The data stored in the microSD memory shown in Figure 1b were treated by a moving average filter with an N length window, which allows smoothing of the signal obtained from the potential difference measured in the half-cell.
The most significant data captured at different concentrations are shown in Figure 2, in which it is observed that the signals reached a stable state in the potential difference of each, which can be used for their subsequent characterization. The stabilization of the voltage occurs approximately in the same period, after the ionic migration of the oxidation-reduction reaction (transient response).
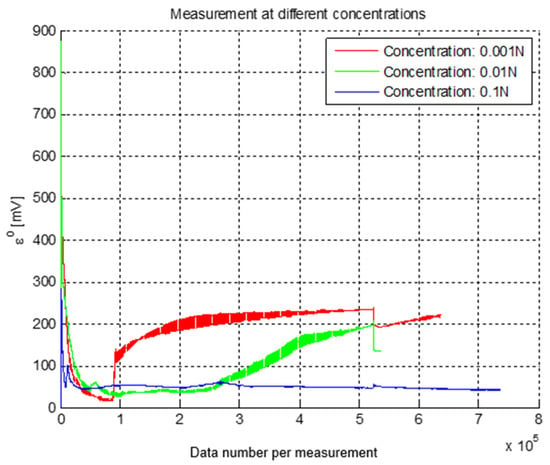
Figure 2.
Graph of emf measurement at different concentrations of NaCl.
The data show the behavior of the concentration and the emf obtained for each concentration [5,15]. A scale was established to observe the change in electric potential as a function of the ionic activity in the solution [16].
Figure 3 shows that the values of the half-cell (Ɛ°) are proportional to the logarithm of the ion concentration in the solution, in addition to presenting a Nernstian slope, as shown in equation 7, which presents an r2 coefficient of 0.9953 and a correlation of 0.99.
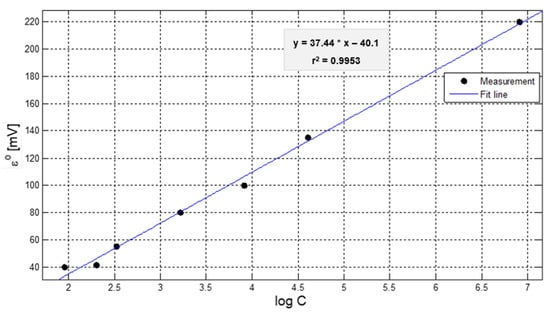
Figure 3.
Graph of half-cell values (Ɛ°) as a function of the logarithm of the concentration.
It is important to mention that the value of the half-cell (Ɛ° = 1.2126 V) corresponds to the standard electrode data and confirms a reversible reaction and the ion selectivity; while the value of the half-cell l (Ɛ° = −40.47) represents the value of the ion that will be detected in the analyte.
It is of note that it was possible to optimize the resolution of the analog-digital converter of the ATMEGA 328P microcontroller so that it can detect potential differences of 1.05 mV, in addition to increasing the number of samples per second that are processed through this internal microcontroller module.
4. Conclusions
In this study, it was possible to implement a novel chloride ion measurement system using a selective membrane (natural) and a support structure to assemble a half-cell based on a design made in a 3D printer. The half-cell obtained Ɛ° = 1.2126 V; this data corresponds to the value reported for a standard electrode, ion-selective and for a reversible reaction. The indicated value of the ion-selective in the half cell (Ɛ° = −40.47), represents chloride ions (Cl−).
The behavior of ionic activity at different concentrations shows a logarithmic trend and a typical Nernstian response and is verified with the coefficient (r2 of 0.9953) of the fitted curve.
Based on the measurements made, the results obtained and the functionality of the assembled device, it was determined that the electrode can be used as a potential chloride ion meter for wastewater and drinking water.
Author Contributions
Conceptualization, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; methodology, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; software, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; validation, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; formal analysis, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; investigation, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; resources, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; data curation, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; writing—original draft preparation, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; writing—review and editing, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; visualization, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; supervision, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; project administration, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A.; funding acquisition, D.A.T.-G., H.R.-G., L.E.C.-G. and E.G.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We want to thank Nicolás Flores Sámano and for help in the design and 3D printing, Roxana Robles García and María Guadalupe Martínez Muñoz for their help in the laboratory work and experimental tests, and finally Diana Priscila Vicencio Toriz for the English revision.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Arada-Pérez, M.; Yazdani-Pedram, M.; Pérez-Saavedra, J. Sensores electroquímicos basados en sales cuaternarias de amonio. Rev. Cuba. Química 2008, 20, 31–38. [Google Scholar]
- Xiao, K.P.; Bühlmann, P.; Nishizawa, S.; Amemiya, S.; Umezawa, U. A chloride ion-selective solvent polymeric membrane electrode based on a hydrogen bond forming ionophore. Anal. Chem. 1997, 69, 1038–1044. [Google Scholar] [CrossRef]
- Meyerhoff, M.E.; Opdycke, W.N. Ion-Selective Electrodes. Adv. Clin. Chem. 1986, 25, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Olmos, R.; Rios, A.; Fernández, J.R.; Lapa, R.A.S.; Lima, J.L.F.C. Construction and evaluation of ion selective electrodes for nitrate with a summing operational amplifier. Appl. Tob. Análisis. Talanta. 2001, 53, 741–748. [Google Scholar] [CrossRef]
- Midgley, D. Limits of detection of ion-selective electrodes: Theory and practice. Trans. Inst. Meas. Control 1987, 9, 25–36. [Google Scholar] [CrossRef]
- Gómez-Biedma, S.; Soria, M. Análisis electroquímico. Rev. Diagn. Biol. 2002, 51, 18–27. [Google Scholar]
- Ewing, G.W. Métodos Instrumentales de Análisis Químico; Mc Graw Hill: New York, NY, USA, 1978; pp. 282–369. ISBN 968-6046-10-0. [Google Scholar]
- Levine, I.N. Fisicoquímica, 5th ed.; Mc Graw Hill Interamérica: Aravaca Madrid, Spain, 2004; pp. 515–571. ISBN 84-481-3787-6. [Google Scholar]
- Maron, S.H.; Prutton, C.F. Fundamentos de Fisicoquímica; Limusa: Johannesburg, South Africa, 2012; pp. 479–553. ISBN 968-18-0164-4. [Google Scholar]
- Eggers, D.F.; Gregory, N.W.; Halsey, G.D.; Rabinovitch, B.S. Fisicoquímica; Limusa Wiley: Mexico City, México, 1967; ISBN 0471064998. [Google Scholar]
- Arada-Pérez, M.A.; Pérez-Marín, L.; Calvo-Quintana, J.; Alonso-Chamarro, J.; Tacoronte-Morales, J. Evaluación de un electrodo selectivo a nitrato de membrana líquida con cloruro de trioctilmetilamonio sobre un soporte conductor. Rev. Mex. De Ing. Química 2002, 1, 23–28. [Google Scholar]
- Creus, S.A. Instrumentación Industrial, 8th ed.; Alfaomega Editores: Ciudad de México, México, 2011; pp. 370–376. ISBN 978-607-707-042-9. [Google Scholar]
- Toriz, G.D.A.; Loredo, A.R.; Marcial, M.D. Elaboración de Electrodo para medición de Cloruros en agua. Acad. J. 2013, 5, 3544–3549. [Google Scholar]
- Somoza-Chuay, J.A.; Pavoni-Oliver, S.; Eirez-Izquierdo, J.E.; Bistel-Esquivel, R.A. Análisis de respuestas potenciométricas de electrodos de Vidrio/ITO y Vidrio/ITO/PANI-LS en la medición de pH. Ing. Electrónica Automática Y Comun. 2019, 40, 1–9. [Google Scholar]
- Ben-Rayana, M.C.; Burnett, R.W.; Covington, A.K.; D’Orazio, P.; Fogh-Andersen, N.; Jacobs, E.; Kataky, R.; Külpmann, W.R.; Kuwa, K.; Larsson, L.; et al. Recommendation for measuring and reporting chloride by ISEs in undiluted serum, plasma or blood. Clin. Chem. Lab. Med. 2006, 44, 346–352. [Google Scholar] [CrossRef]
- de Vera, G.; Climenta, M.A.; Antón, C.; Hidalgo, A.; Andrade, C. Determination of the selectivity coefficient of a chloride ion selective electrode in alkaline media simulating the cement paste pore solution. J. Electroanal. Chem. 2010, 639, 43–49. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).