An Inkjet-Printed Amperometric H2S Sensor for Environmental Applications †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inkjet-Printed H2S Sensor Fabrication
2.2. Sensor Characterization
3. Results and Discussion
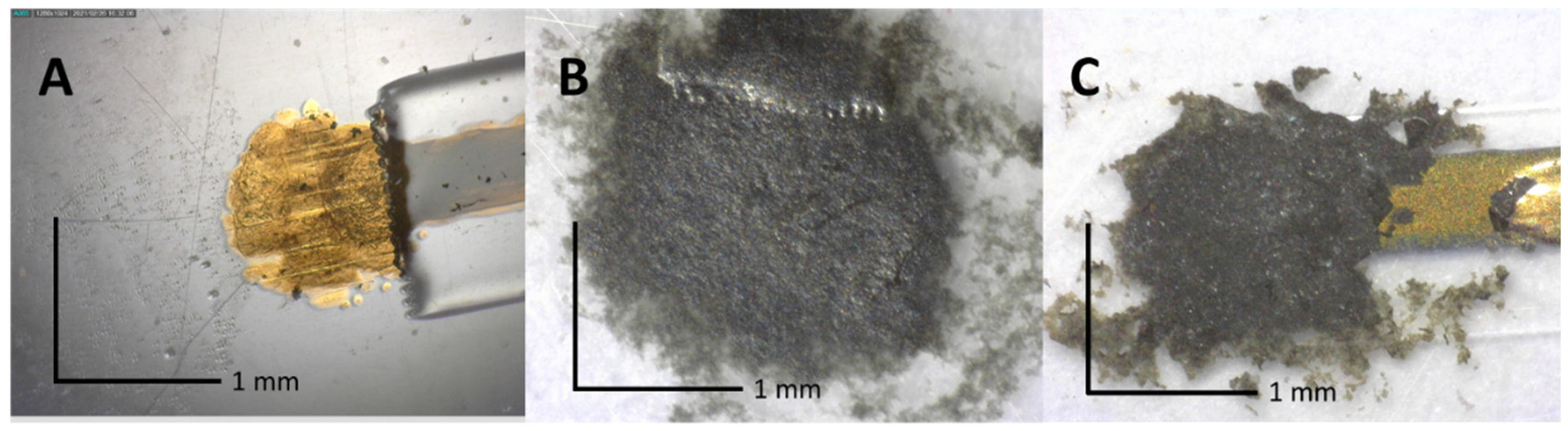
3.1. Inkjet-Printed H2S Miniaturized Sensor Fabrication
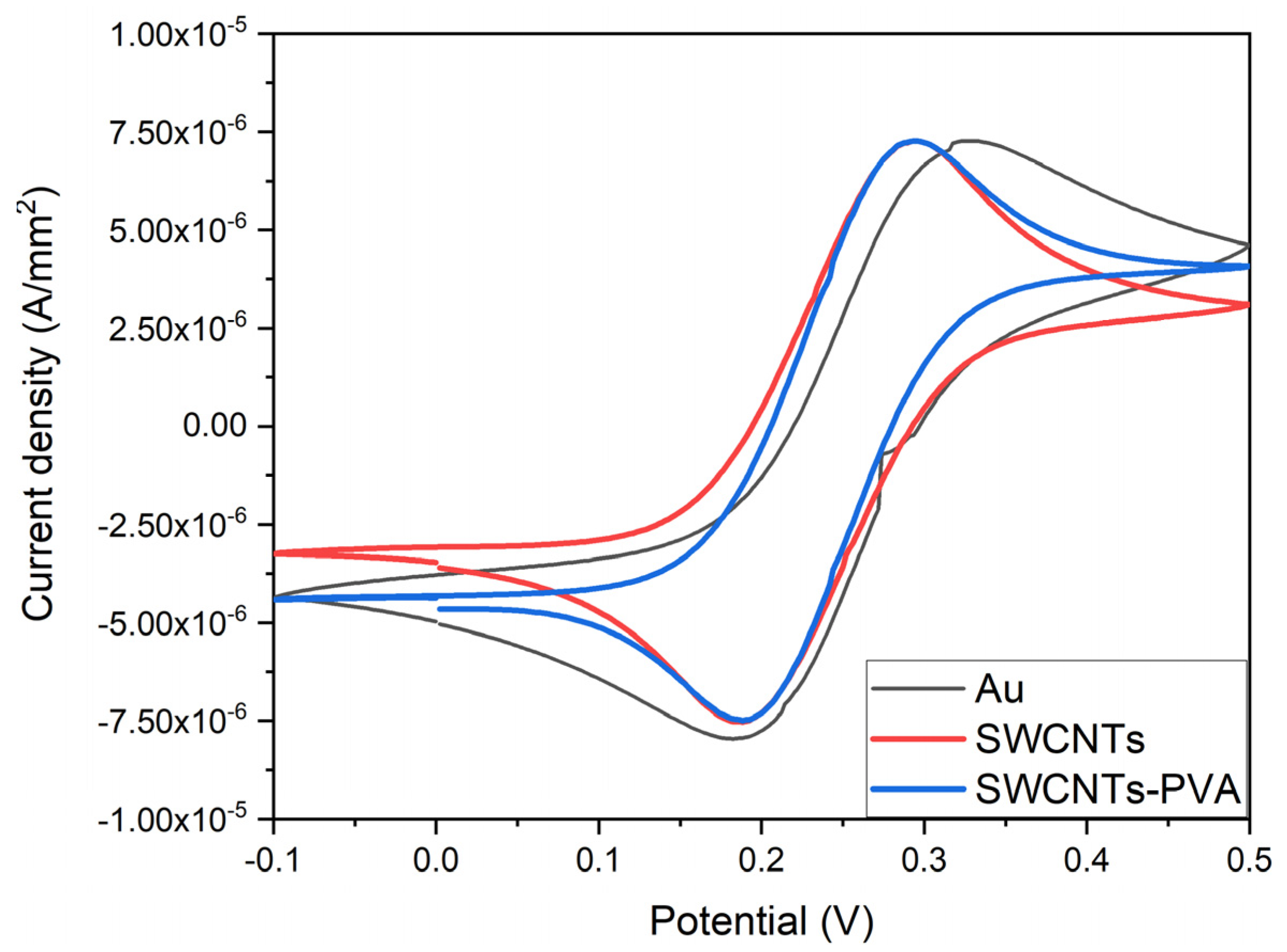
3.2. Microsensor Electrochemical Characterization
3.3. PVA Deposition Temperature Study
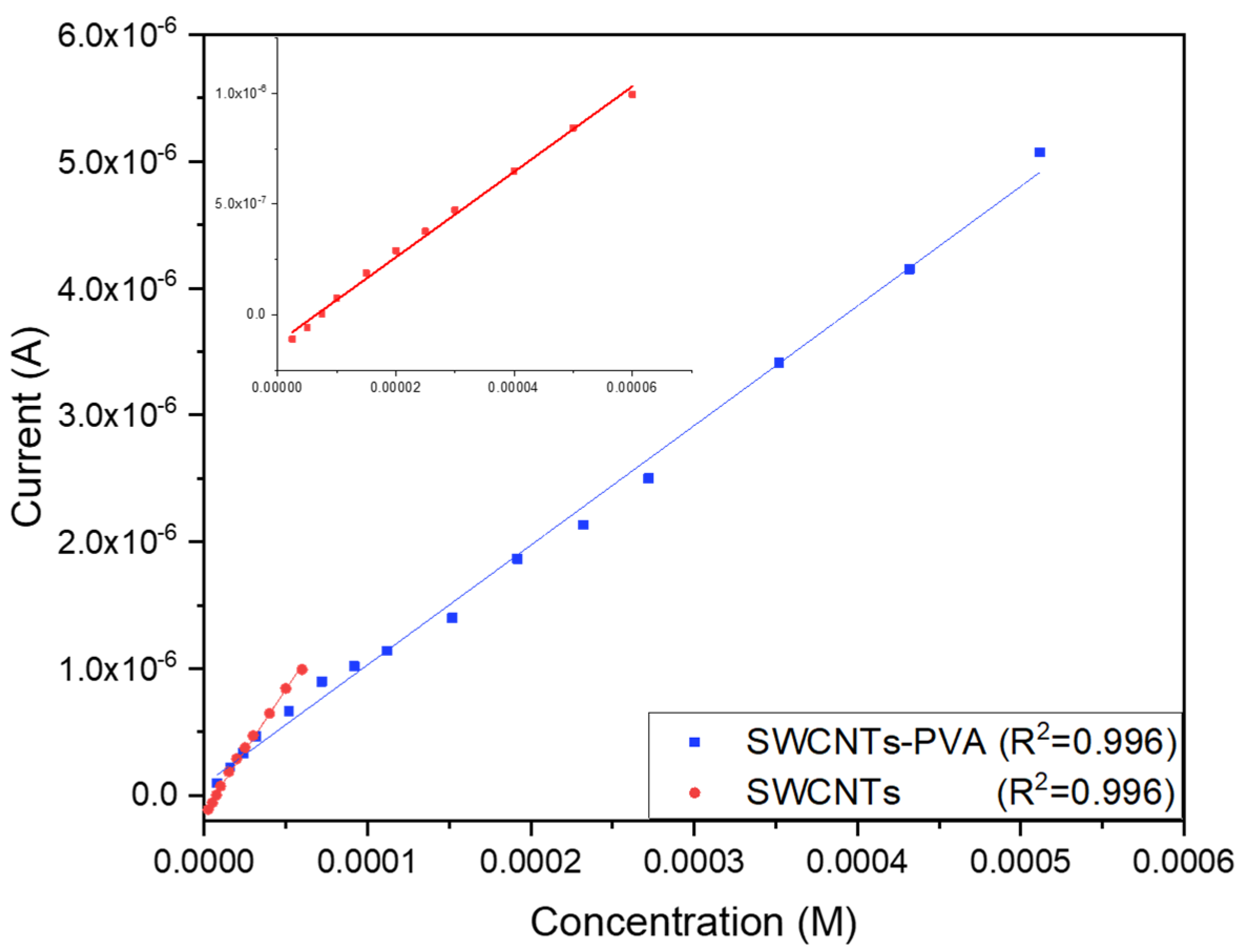
3.4. Microsensor Calibration and Analytical Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Li, C.; Zhang, M. Exploring the spatial spillover effects of industrialization and urbanization factors on pollutants emissions in China’s Huang-Huai-Hai region. J. Clean. Prod. 2018, 195, 154–162. [Google Scholar] [CrossRef]
- Aikawa, M.; Hiraki, T.; Suzuki, M.; Tamaki, M.; Kasahara, M. Separate chemical characterizations of fog water, aerosol, and gas before, during, and after fog events near an industrialized area in Japan. Atmos. Environ. 2007, 41, 1950–1959. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen Sulfide. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Soreanu, G.; Falletta, P.; Béland, M. Removal of Hydrogen Sulfide from Gas Streams Using Biological Processes—A Review. Can. Biosyst. Eng. 2006, 48, 2. [Google Scholar]
- Moya, A.; Gabriel, G.; Villa, R.; del Campo, F.J. Inkjet-printed electrochemical sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Wang, J.; Hu, P.; Jiang, Z. Magnetic MoS2 on multiwalled carbon nanotubes for sulfide sensing. Anal. Chim. Acta 2017, 975, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Schoenfisch, M.H. Direct Electrochemical Sensing of Hydrogen Sulfide without Sulfur Poisoning. Anal. Chem. 2018, 90, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Hao, X.; Song, Y.; Liang, X.; Liu, F.; Liu, F.; Sun, P.; Gao, Y.; Yan, X.; et al. Nafion-based amperometric H2S sensor using Pt-Rh/C sensing electrode. Sens. Actuators B Chem. 2018, 273, 635–641. [Google Scholar] [CrossRef]
- Brown, M.D.; Hall, J.R.; Schoenfisch, M.H. A direct and selective electrochemical hydrogen sulfide sensor. Anal. Chim. Acta 2019, 1045, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Deo, R.P.; Wang, J. Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes. Anal. Chim. Acta 2004, 517, 131–137. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Hacker, V. Preparation and Characterization of PVA/PDDA/Nano-Zirconia Composite Anion Exchange Membranes for Fuel Cells. Polymers 2019, 11, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, J.; Song, Z.; Liu, X.; Lü, W.; Li, X. Fabrication and Electrochemical Performance of PVA/CNT/PANI Flexible Films as Electrodes for Supercapacitors. Nanoscale Res. Lett. 2020, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tao, X.; Xue, P.; Cheng, X. Enhanced mechanical properties and morphological characterizations of poly(vinyl alcohol)–carbon nanotube composite films. Appl. Surf. Sci. 2005, 252, 1404–1409. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods: For the Examination of Water and Wastewater, 22th ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
| Temperature | Ip Au Electrode (µA) | Ip Au Electrode + PVA (µA) | % Ip Reduction |
|---|---|---|---|
| 25 °C | 8.12 | 7.46 | 8.1 |
| 90 °C | 3.92 | 1.22·10−5 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paré, F.; Castro, R.; Guimera, X.; Gabriel, G.; Baeza, M. An Inkjet-Printed Amperometric H2S Sensor for Environmental Applications. Chem. Proc. 2021, 5, 4. https://doi.org/10.3390/CSAC2021-10462
Paré F, Castro R, Guimera X, Gabriel G, Baeza M. An Inkjet-Printed Amperometric H2S Sensor for Environmental Applications. Chemistry Proceedings. 2021; 5(1):4. https://doi.org/10.3390/CSAC2021-10462
Chicago/Turabian StyleParé, Franc, Rebeca Castro, Xavier Guimera, Gemma Gabriel, and Mireia Baeza. 2021. "An Inkjet-Printed Amperometric H2S Sensor for Environmental Applications" Chemistry Proceedings 5, no. 1: 4. https://doi.org/10.3390/CSAC2021-10462
APA StyleParé, F., Castro, R., Guimera, X., Gabriel, G., & Baeza, M. (2021). An Inkjet-Printed Amperometric H2S Sensor for Environmental Applications. Chemistry Proceedings, 5(1), 4. https://doi.org/10.3390/CSAC2021-10462






