1. Introduction
Aged distilled beverages, namely cognac and brandy, contain natural phenolic antioxidants that are often considered as quality markers for these beverages [
1]. Phenolic acid (gallic and ellagic) and aromatic aldehydes (vanillin and syringaldehyde) are the major contributors to the antioxidant properties of aged distilled beverages [
1,
2,
3]. Their simultaneous determination is of practical interest.
Different types of chromatography and capillary electrophoresis are usually applied for their quantification [
2,
4,
5]. Nevertheless, less tedious and simpler methods are encouraged. Electrochemical sensors are simple, reliable, and cost-effective and could be an effective alternative tool for these purposes [
6,
7,
8]. The only problem facing electrochemical approaches is their low selectivity of when determining the phenolic content due to the structural similarity of the analytes. This limitation can be overcome using chemically modified electrodes. Thus, electrodes based on a Printex L6 carbon-silver hybrid nanomaterial [
9], layer-by-layer combination of multi-walled carbon nanotubes (MWCNT), and electropolymerized quercetin [
10] or gallic acid [
11], reduced graphene oxide, and poly(glutamic acid) [
12] were developed for the determination of gallic acid. An MWCNT-based sensor is described for the ellagic acid quantification [
3]. Syringaldehyde is not usually considered for electroanalysis, while many electrochemical sensors have been developed for vanillin determination, including ones that are based on a combination of carbon nanomaterials and electropolymerized films [
13,
14].
Nevertheless, the simultaneous voltammetric detection of gallic and ellagic acids as well as aromatic aldehydes not often considered. Currently, there is just one example of gallic and ellagic acid quantification in a mixture using an MWCNT-modified carbon paste electrode [
6]. The possibility of the simultaneous determination of syringaldehyde and vanillin has been demonstrated on a glassy carbon electrode (GCE) that was modified with carbon nanofibers and cationic surfactant cetylpyridinium bromide [
7]. The analytical characteristics presented in
Table 1 can be improved further. The disadvantage of both approaches is the absence of data on the selectivity of the electrode response to the target analytes. Furthermore, the use of this application in real samples was not realized.
Thus, novel voltammetric sensors that are based on carbon nanotubes and electropolymerized pyrocatechol violet (PCV) or p-aminobenzoic acid (ABA) have been developed for the simultaneous determination of phenolic antioxidants in cognac and brandy for the first time.
2. Materials and Methods
PCV and 99% ABA from Sigma-Aldrich (India and Germany, respectively) were used as monomers to obtain polymeric coverage. Their standard solutions (10 mM for PCV and 25 mM for ABA) were prepared in distilled water. Gallic (99%) and ellagic (95%) acids, vanillin (99%) from Sigma (Germany), and syringaldehyde (98%) from Aldrich (Germany) were used as standards. Their 10 mM (0.86 mM for ellagic acid) stock solutions in c.p. grade methanol (ethanol (rectificate) in the case of aromatic aldehydes) were prepared in 5.0 mL flasks. The exact dilution with the corresponding solvent was used for the preparation of less concentrated solutions.
Britton–Robinson buffer with a pH of 2.0 was used as a supporting electrolyte for the quantification of the natural phenolics. It was prepared from a mixture of 0.04 M boric acid, 0.04 M phosphoric acid, and 0.04 M acetic acid. The pH value was adjusted using a 0.2 M NaOH solution on a pH meter.
MWCNT (outer diameter 40–60 nm, inner diameter 5–10 nm and 0.5–500 μm length) and polyaminobenzene sulfonic acid functionalized single-walled carbon nanotubes (f-SWCNT) (d × l is 1.1 nm × 0.5–1.0 μm) were purchased from Aldrich and Sigma-Aldrich (Steinheim, Germany), respectively. MWCNT (0.5 mg mL−1 homogeneous suspension in 1% sodium dodecylsulfate (Panreac, Barcelona, Spain) was prepared by sonication for 30 min in an ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Korea). A homogeneous 1.0 mg mL−1 suspension of f-SWCNT was obtained by ultrasonic dispersion for 30 min in dimethylformamide.
All reagents were chemical grade purity. Double distilled water was used for the measurements. The experiments were conducted at laboratory temperature (25 ± 2 °C).
Voltammetric measurements were conducted on the potentiostat/galvanostat Autolab PGSTAT 12 (Eco Chemie B.V., Utrecht, The Netherlands) with GPES software, version 4.9.005. Electrochemical impedance spectroscopy (EIS) was performed on the potentiostat/galvanostat Autolab PGSTAT 302N with the FRA 32M module (Eco Chemie B.V., Utrecht, The Netherlands) and NOVA 1.10.1.9 software. The 10 mL glassy electrochemical cell consisted of the working GCE with a 7.07 mm2 geometric surface area (CH Instruments, Inc., Bee Cave, TX, USA), or a modified electrode, a silver-silver chloride saturated KCl reference electrode, and a platinum wire as the counter electrode was used.
An “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russian Federation) equipped with a glassy electrode was applied for the pH measurements.
Scanning electron microscopy (SEM) was conducted on a high-resolution field emission scanning electron microscope MerlinTM (Carl Zeiss, Oberkochen, Germany) at the accelerating voltage of 5 kV and at the emission current of 300 pA.
3. Results and Discussion
3.1. Polymer-Based Sensors Creation and Their Characteristics
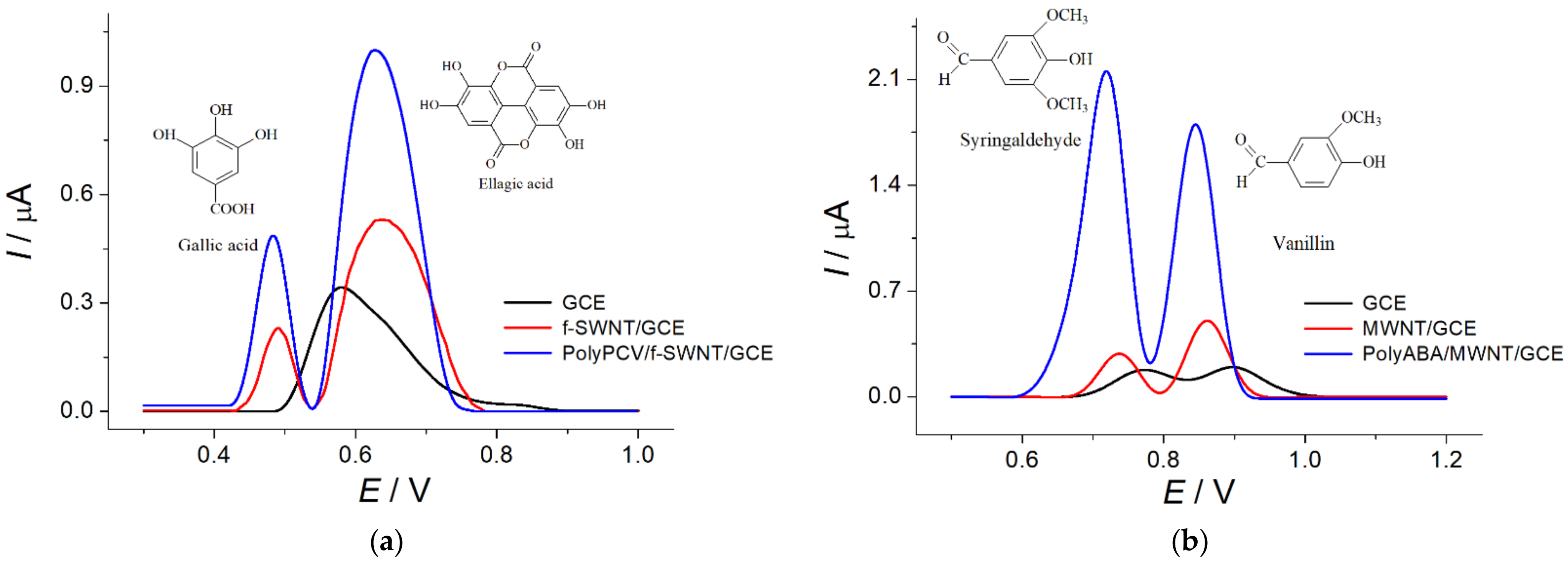
A carbon nanotube-modified GCE was used as a substrate for polymeric coverage and electrochemical deposition. For this reason, f-SWCNT/GCE and MWCNT/GCE were obtained by drop-casting 2.0 or 4.0 μL of the f-SWCNT or MWCNT suspension, respectively, at the GCE surface. The application of the carbon nanotubes provided high surface area and conductivity for the electrode. Then, the electropolymerization of PCV and ABA in potentiodynamic mode was performed. Both PCV and ABA form conductive polymers, the formation of which were confirmed by the appearance of quasi-reversible redox peaks on the cyclic voltammograms. The redox currents are increased when the number of cycles is grown. The polymerization conditions of PCV and ABA were optimized based on the voltammetric response of the pairs of target analytes (gallic and ellagic acids on the polyPCV/f-SWCNT/GCE and syringaldehyde and vanillin on the polyABA/MWCNT/GCE). The peak potential separation for both types of analytes did not change, regardless of whether it was on the polymer-based sensors vs. the carbon nanotube-modified electrodes (
Figure 1), while oxidation currents showed a statistically significant increase. Thus, the electropolymerization of PCV should be performed using a 50 μM monomer in 0.1 M H
2SO
4 with 10 cycles ranging from −0.2 to 1.1 V at 50 mV s
−1. The optimum conditions for ABA electropolymerization are 20-fold potential cycling from −0.5 to 2.0 V at 100 mV s
−1 from a 100 μM monomer in Britton–Robinson buffer pH 2.0.
The sensors that were developed have been characterized with scanning electron microscopy (SEM), cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS). According to SEM data, the polymeric coverages exhibit a porous structure, with the shape of the particles and their aggregates deposited on the surface of the carbon nanomaterials confirming that electropolymerization was successful (
Figure 2).
The electroactive surface area of the electrodes was calculated on the basis of cyclic voltammetry and chronoamperometry (for GCE) in the presence of 1.0 mM [Fe(CN)6]4−. A statistically significant increase in the effective surface area (49.0 ± 0.2 mm2 for polyPCV/f-SWCNT/GCE and 89 ± 4 mm2 for polyABA/MWCNT/GCE vs. 38.9 ± 0.6 mm2 for f-SWCNT/GCE, 75 ± 3 mm2 for MWCNT/GCE, and 8.2 ± 0.3 mm2 for GCE) explain the increase in the analyte oxidation currents well. The EIS data show that the polymer-based sensors demonstrate lower charge transfer resistance (26.0 ± 0.4 and 4.9 ± 0.3 kΩ for polyPCV/f-SWCNT/GCE and polyABA/MWCNT/GCE) compared to the GCE (68 ± 4 and 72 ± 3 kΩ) and the GCE modified with carbon nanotubes (19.2 ± 0.8 kΩ for f-SWCNT/GCE and 12.1 ± 0.9 kΩ for MWCNT/GCE). Thus, polymer-based sensors are characterized by a higher electron transfer rate. SEM and electrochemical method data confirm the effectivity of the developed modifiers.
3.2. Simultaneous Quantification of Natural Phenolic Antioxidants
For the quantification of the phenolics under consideration, the sensors that were created were used under differential pulse voltammetry conditions in Britton–Robinson buffer medium with a pH of 2.0 in order to provide the highest possible oxidation currents for the analytes (phenolic acids and aromatic aldehydes). The linear dynamic ranges of 0.75–10 and 10–100 μM for gallic acid and 0.75–7.5 and 7.5–100 μM for ellagic acid on the polyPCV-based sensor were obtained. The detection limits were equal to 0.12 μM for gallic acid and 0.11 μM for ellagic acid. The PolyABA-based sensor provided linear response in the ranges of 0.075–7.5 and 7.5–100 μM for syringaldehyde and 0.50–7.5 and 7.5–100 μM for vanillin when the detection limits were 0.018 and 0.19 μM, respectively. The detection limits were calculated as 3SD
a/b, where SD
a is the standard deviation of the intercept of the calibration graph and where b is the slope of the calibration graph. The analytical characteristics that were obtained are improved vs. those of other modified electrodes [
6,
7]. The proven independent electrooxidation of the analyte pairs allows the application of calibration graphs for the equimolar mixtures, which is another advantage of the approaches developed here. The high accuracy of the sensors has been confirmed by recovery values (97.1–101%) that were determined using model solutions for the studied phenolics. The relative standard deviation values of 0.41–4.8% indicate that the sensor response has high reproducibility as long as a new electrode is prepared before each measurement.
The sensor selectivity in the presence of typical interferences and other natural phenolics was obtained, which is an important advantage. The 1000-fold excess of inorganic ions (K+, Mg2+, Ca2+, NO3‾, Cl‾, and SO42), glucose, rhamnose, and sucrose as well as ascorbic acid (1000- and 100-fold excess in the case of phenolic acids and aromatic aldehydes, respectively) does not show an interference effect. It has been proven that there is an absence of an interference effect caused by syringaldehyde and vanillin (<2.5 and <10 μM, respectively) on the oxidation peaks of phenolic acids. On contrary, gallic and ellagic acid are the major potential causes of interference for the determination of the aromatic aldehydes that are under consideration. A 10-fold excess of gallic acid and <1.0 μM of ellagic acid do not interfere with the determination of vanillin and syringaldehyde. The interference effect of ellagic acid caused by its high concentration in cognac and brandy can be excluded via sample dilution. Thus, the sensors that have been developed here can be applied for the analysis of aged distilled beverages.
3.3. Real Samples Analysis
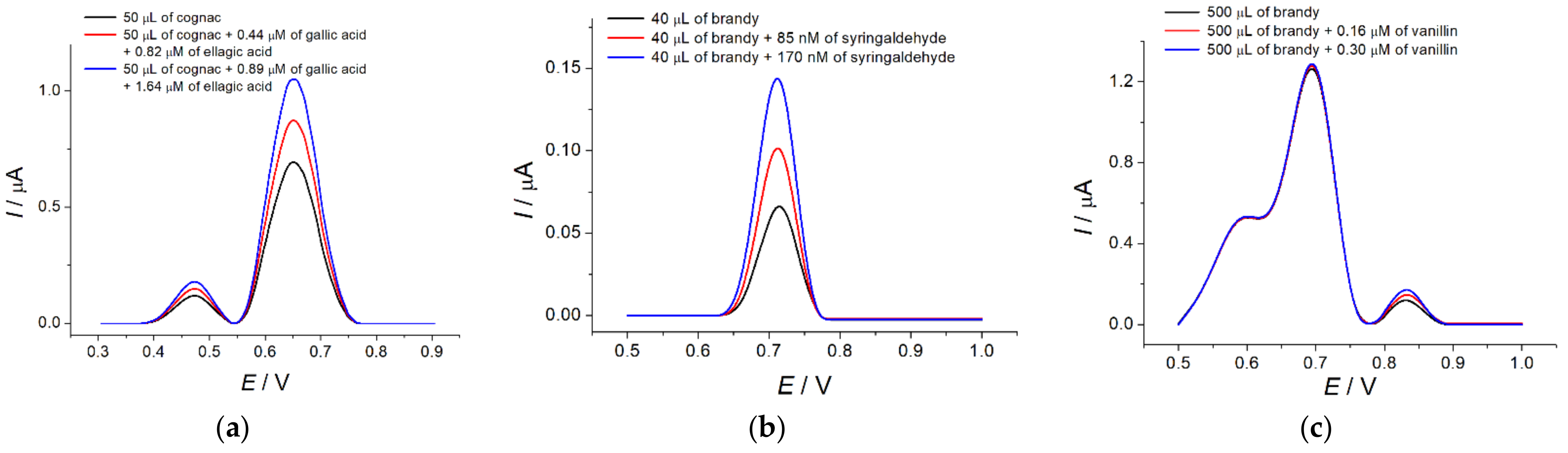
The sensors that were developed were successfully tested on cognac and brandy samples. The phenolic acids and aromatic aldehydes show resolved oxidation peaks on the differential pulse voltammograms for cognac and brandy that is confirmed by the standard addition method (
Figure 3). Due to the low concentration of vanillin, the sample volume varied for the quantification of syringaldehyde and vanillin (40 and 500 μL, respectively). The recovery rate of 98.9–102% indicates the absence of matrix effects in these determinations.
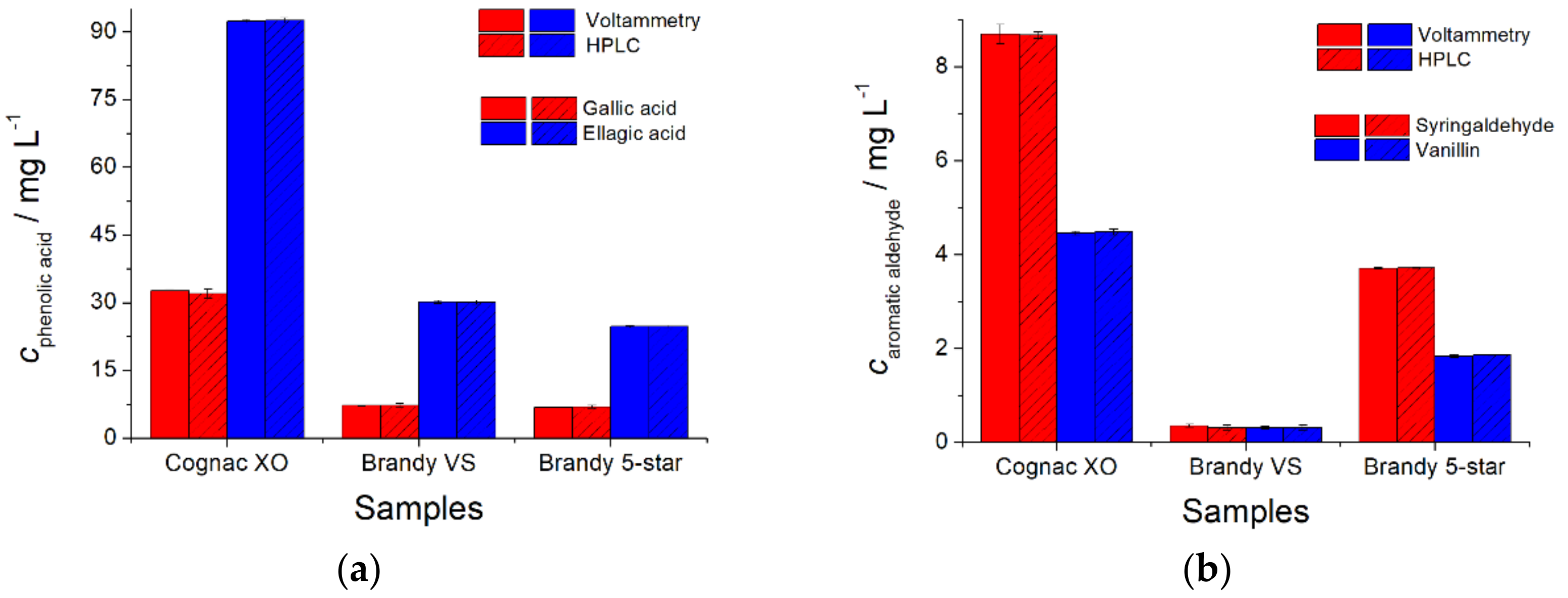
The quantification of natural phenolic antioxidants in cognac and brandy has been performed and compared using an independent chromatographic determination (
Figure 4). The results that were obtained agree with each other well.
t-Test data (0.0100–2.19) are less than the critical value of 2.45, which indicates the absence of systematic errors in the determination. Similarly, the
F-test results (1.00–17.36) achieved the less than critical value of 19.25, indicating that the methods that were used in this study demonstrate uniform precision.
Thus, the novel highly sensitive and selective voltammetric sensors that were developed in this study for the simultaneous determination of the structurally related phenolic antioxidants in cognac and brandy are characterized by the simplicity of their fabrication as well as their reliability and cost-efficiency, and they can be applied for routine analysis as an alternative to chromatographic methods.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z. and E.G.; investigation, E.G.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, G.Z. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Rustam Davletshin (Department of High Molecular and Organoelement Compounds, Kazan Federal University) for the chromatographic measurements and Aleksei Rogov (Laboratory of the Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the SEM measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziyatdinova, G.; Salikhova, I.; Skorobogatova, N.; Chibisova, M.; Budnikov, H. New electrochemistry based approaches to brandy quality evaluation using antioxidant parameters. Food Anal. Methods 2015, 8, 1794–1803. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Hoffman, B.; Yang, J.; Soleas, G.J. Phenolic constituents, furans, and total antioxidant status of distilled spirits. J. Agric. Food Chem. 1999, 47, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Chronoamperometric estimation of cognac and brandy antioxidant capacity using MWNT modified glassy carbon electrode. Talanta 2014, 125, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Belchior, A.P.; Spranger, M.I.; Bruno-de-Sousa, R. High-performance liquid chromatography method for analysis of phenolic acids, phenolic aldehydes, and furanic derivatives in brandies. Development and validation. J. Sep. Sci. 2003, 26, 496–502. [Google Scholar] [CrossRef]
- Panossian, A.; Mamikonyan, G.; Torosyan, M.; Gabrielyan, E.; Mkhitaryan, S. Analysis of aromatic aldehydes in brandy and wine by high-performance capillary electrophoresis. Anal. Chem. 2001, 73, 4379–4383. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Khayatkashani, M.; Motaghedifard, M.H. Simultaneous determination of ellagic and gallic acid in Punica granatum, Myrtus communis and Itriphal formulation by an electrochemical sensor based on a carbon paste electrode modified with multi-walled carbon nanotubes. Anal. Methods 2011, 3, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Kozlova, E.V.; Ziganshina, E.R.; Budnikov, H.C. Application of electrode modified with carbon nanofibers and cationic surfactant for simultaneous voltammetric determination of syringaldehyde and vanillin. Butlerov Commun. 2015, 42, 132–137. [Google Scholar]
- Ziyatdinova, G.; Guss, E.; Morozova, E.; Budnikov, H.; Davletshin, R.; Vorobev, V.; Osin, Y. Simultaneous voltammetric determination of gallic and ellagic acids in cognac and brandy using electrode modified with functionalized SWNT and poly(pyrocatechol violet). Food Anal. Methods 2019, 12, 2250–2261. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Prado, T.M.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Machado, S.A.S. Synergy between Printex nano-carbons and silver nanoparticles for sensitive estimation of antioxidant activity. Anal. Chim. Acta 2016, 926, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Polyquercetin/MWNT-modified electrode for the determination of natural phenolic antioxidants. Electroanalysis 2017, 29, 2610–2619. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Voltammetric determination of polyphenolic content in pomegranate juice using a poly(gallic acid)/multiwalled carbon nanotube modified electrode. Beilstein J. Nanotechnol. 2016, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feminus, J.J.; Manikandan, R.; Narayanan, S.S.; Deepa, P.N. Determination of gallic acid using poly(glutamic acid): Graphene modified electrode. J. Chem. Sci. 2019, 131, 11. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wang, H.; Yang, L.; Zhao, F.; Zeng, B. Sensitively voltammetric determination of vanillin with a molecularly imprinted ionic liquid polymer-carboxyl single-walled carbon nanotubes composite electrode. Int. J. Electrochem. Sci. 2016, 11, 6009–6022. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).