Ternary Oxidized Carbon Nanohorns/TiO2/PVP Nanohybrid as Sensitive Layer for Chemoresistive Humidity Sensor †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
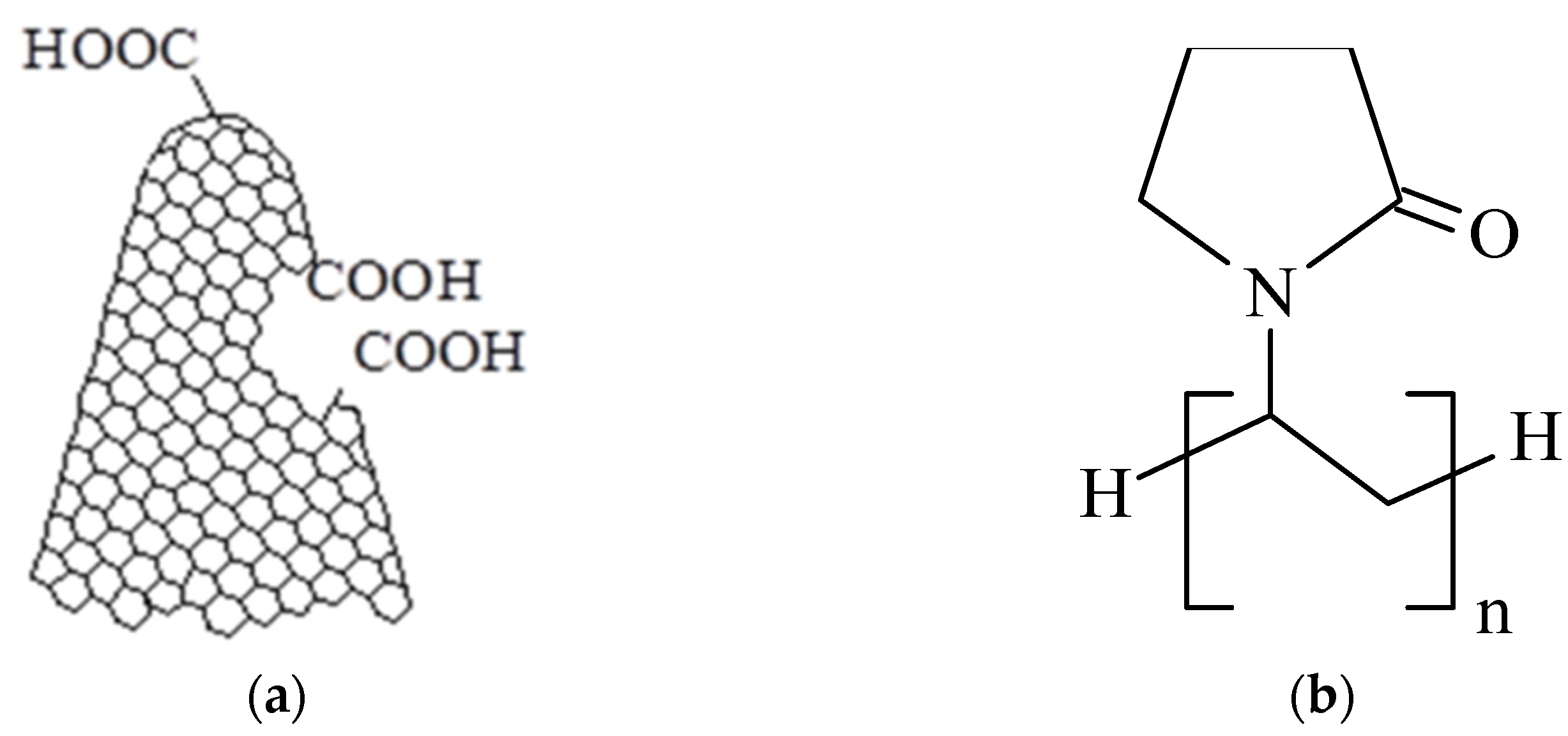
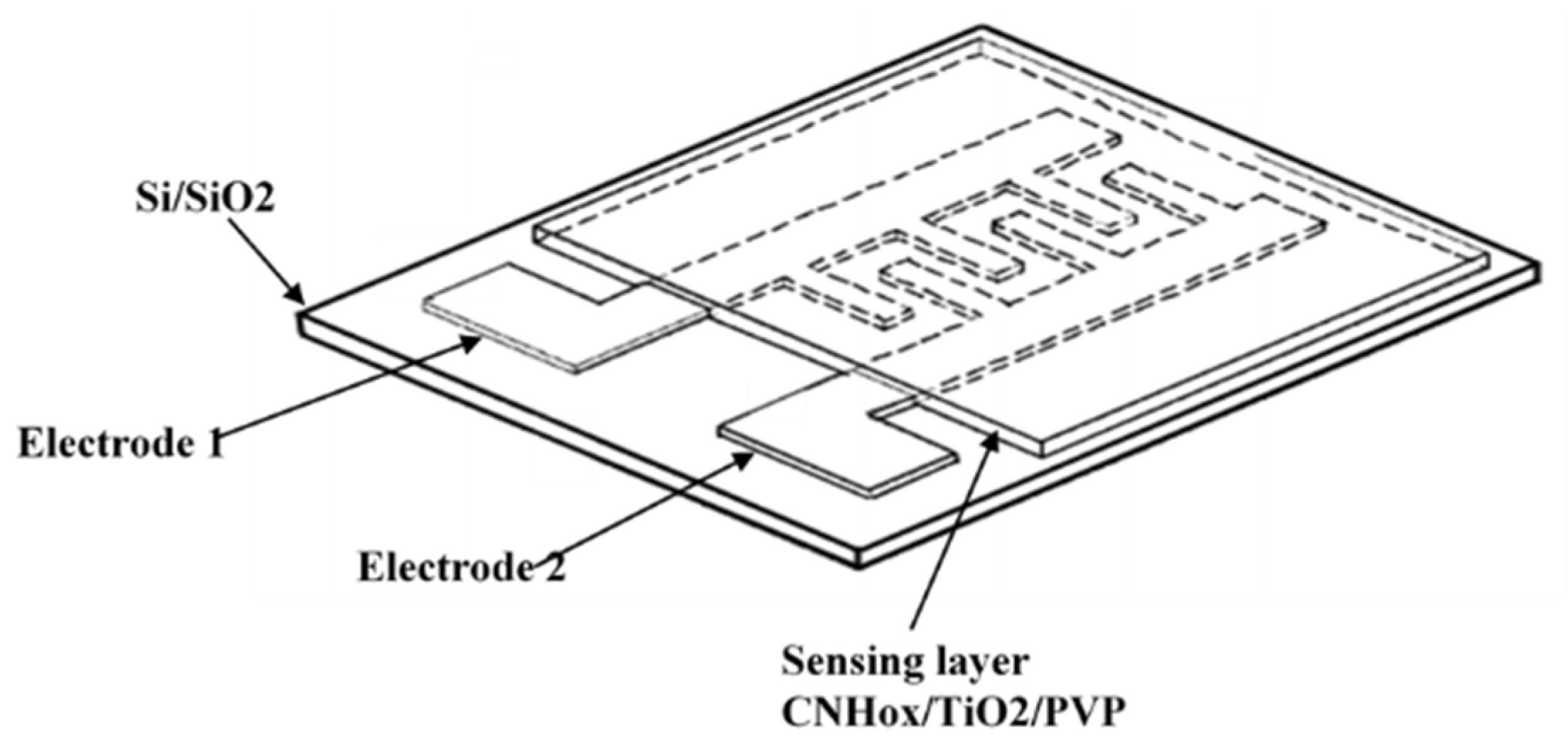
2.2. Synthesis of the Ternary Hybrid Nanocomposite Sensing Films and Experimental Setup
- -
- Heating for 24 h at 90 °C under low pressure (2 mbar);
- -
- Heating for 24 h at 120 °C under low pressure (2 mbar).
3. Results
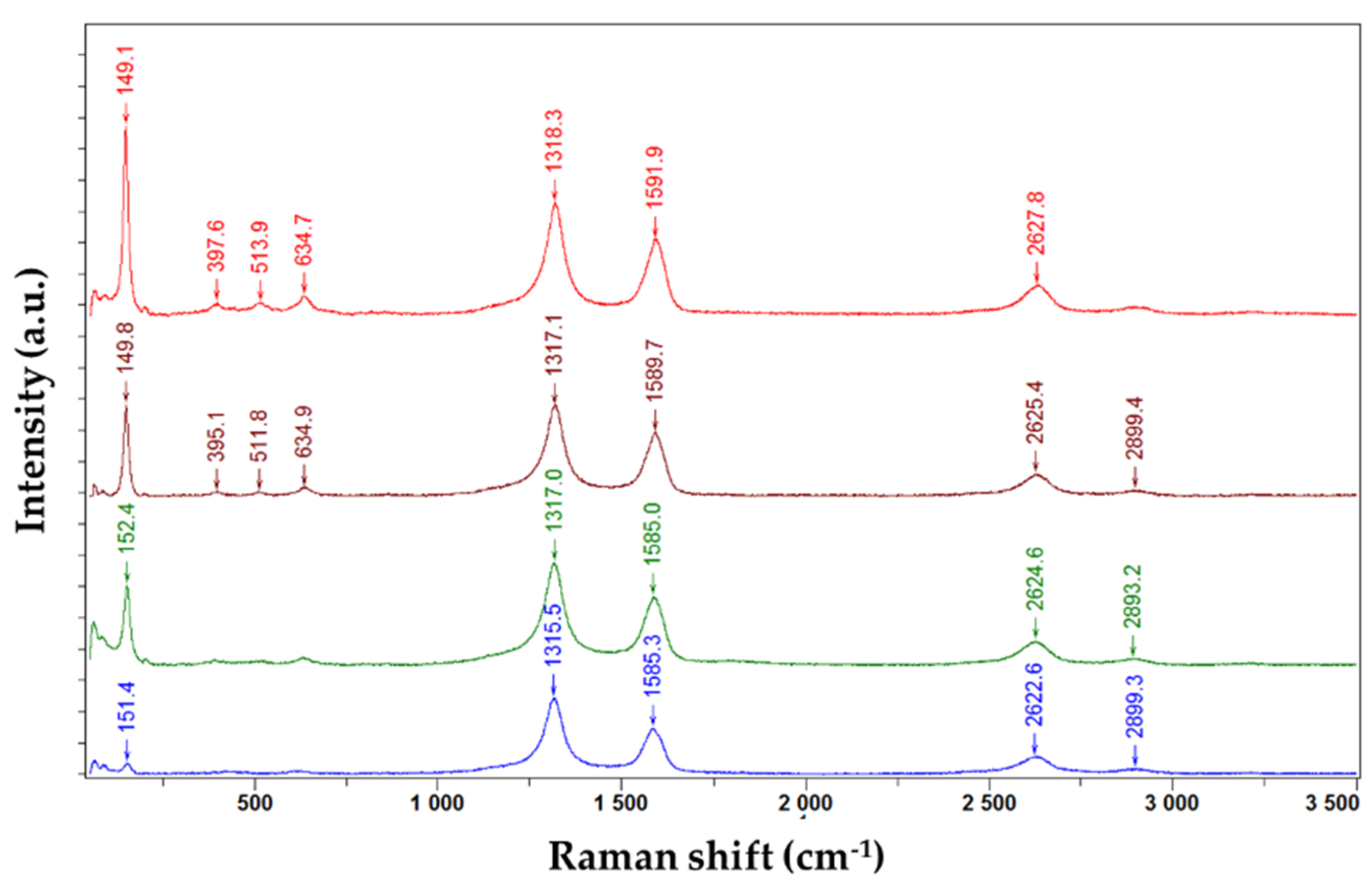
3.1. Raman Spectroscopy
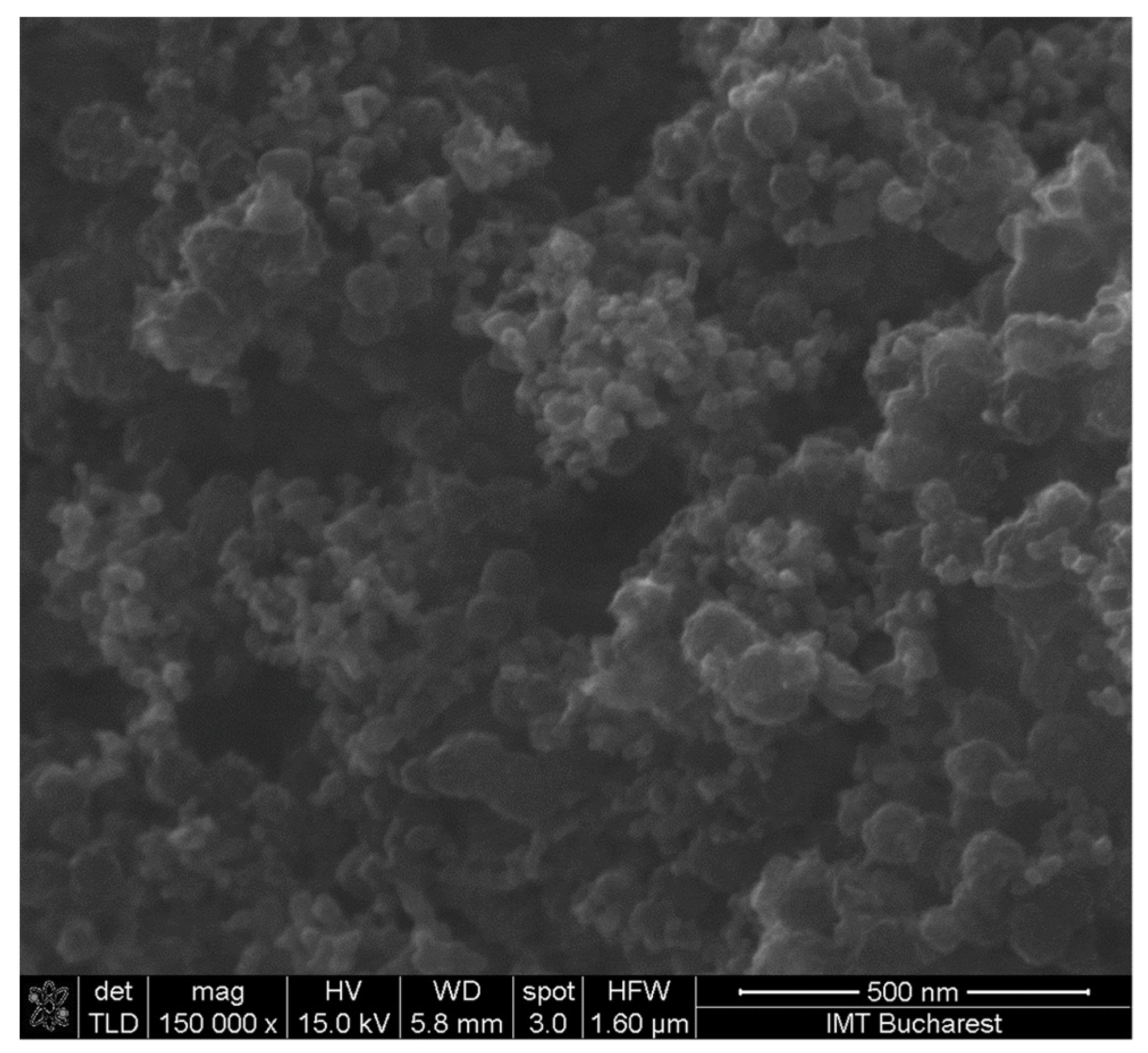
3.2. SEM Analysis
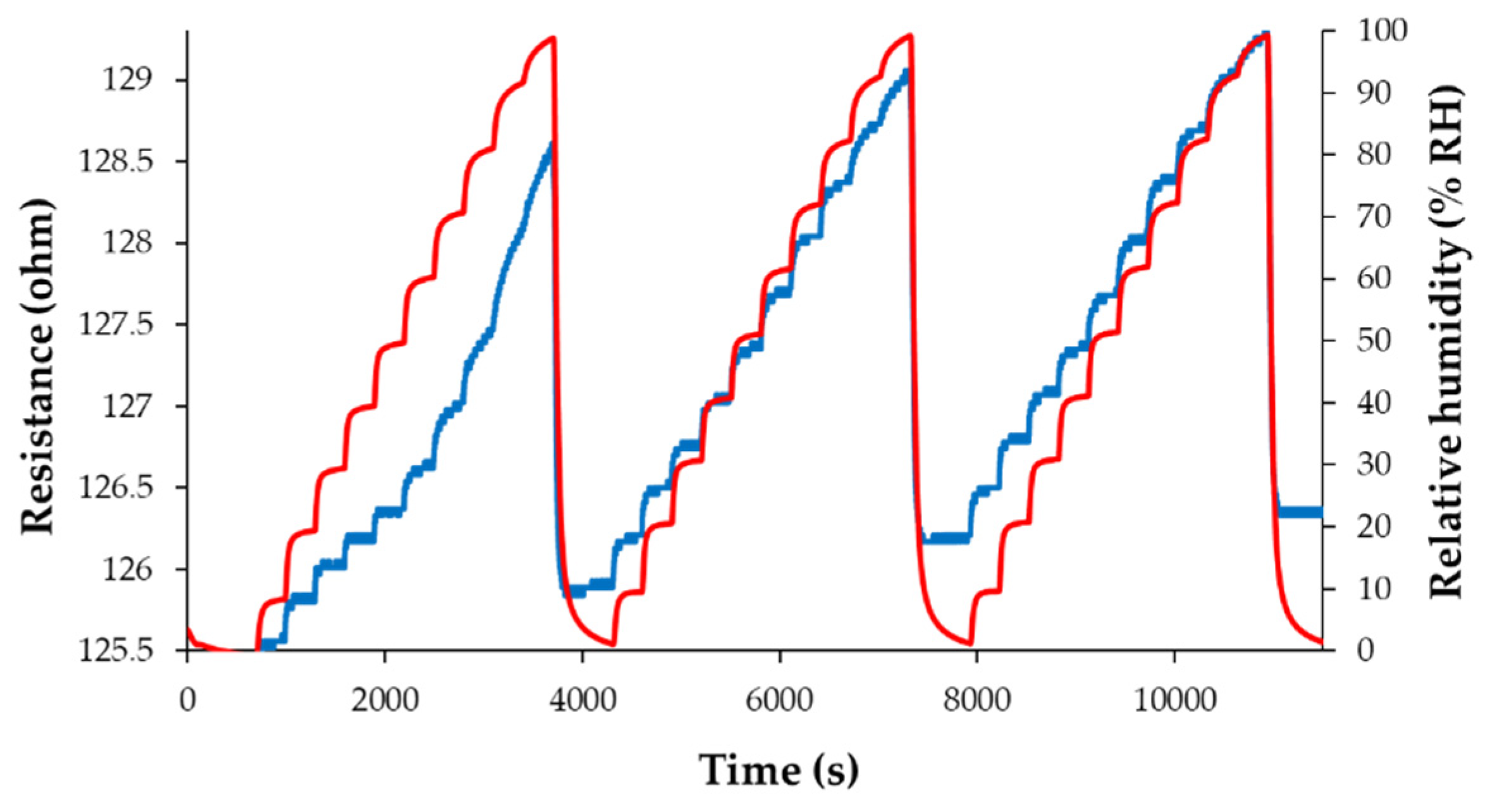
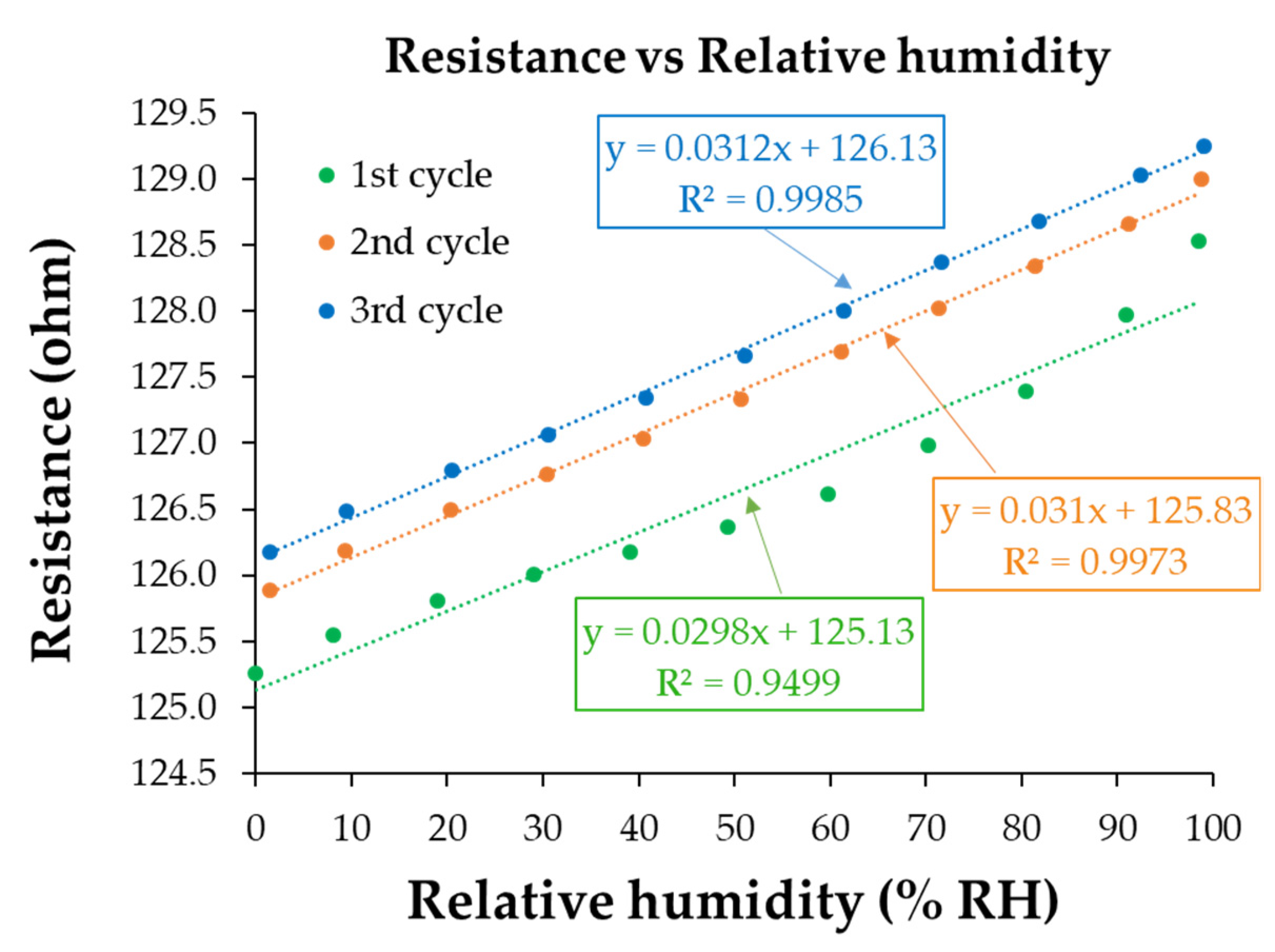
3.3. RH Monitoring Capability of the Ternary Hybrid Nanocomposite
3.4. Analysis of the Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yeo, T.L.; Sun, T.; Grattan, K.T.V. Fibre-optic sensor technologies for humidity and moisture measurement. Sens. Actuators A Phys. 2008, 144, 280–295. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focused review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K. Gas sensing mechanism of TiO2-based thin films. Vacuum 2004, 74, 335–338. [Google Scholar] [CrossRef]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Nicolescu, C.M. Oxidized Carbon Nanohorns as Novel Sensing Layer for Resistive Humidity Sensor. Acta Chim. Slov. 2020, 67, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Pachiu, C.; Nicolescu, C.M. Oxidized Carbon Nanohorn-Hydrophilic Polymer Nanocomposite as the Resistive Sensing Layer for Relative Humidity. Anal. Lett. 2020, 54, 527–540. [Google Scholar] [CrossRef]
- Serban, B.C.; Bumbac, M.; Buiu, O.; Cobianu, C.; Brezeanu, M.; Nicolescu, C. Carbon nanohorns and their nanocomposites: Synthesis, properties, and applications. A concise review. Ann. Acad. Rom. Sci. Ser. Math. Appl. 2018, 11, 5–18. [Google Scholar]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Serbanescu, M.; Pachiu, C.; Serbanescu, M. Electrical Percolation Threshold and Size Effects in Polyvinylpyrrolidone-Oxidized Single-Wall Carbon Nanohorn Nanocomposite: The Impact for Relative Humidity Resistive Sensors Design. Sensors 2021, 21, 1435. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Oxidized Carbon Nanohorns—Based Nanohybrid as Sensing Coating for Room Temperature Resistive Humidity Monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Jagannatham, M.; Berkmans, A.J.; Haridoss, P.; Reddy, L.; Shankar, M.V. Influence of pre-oxidation, versus post-oxidation of carbon nanohorns in TiO2 nanohybrid for enhanced photocatalytic hydrogen production. Mater. Res. Bull. 2019, 109, 34–40. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, B.-C.; Buiu, O.; Bumbac, M.; Marinescu, R.; Dumbravescu, N.; Avramescu, V.; Cobianu, C.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; et al. Ternary Oxidized Carbon Nanohorns/TiO2/PVP Nanohybrid as Sensitive Layer for Chemoresistive Humidity Sensor. Chem. Proc. 2021, 5, 12. https://doi.org/10.3390/CSAC2021-10616
Serban B-C, Buiu O, Bumbac M, Marinescu R, Dumbravescu N, Avramescu V, Cobianu C, Nicolescu CM, Brezeanu M, Radulescu C, et al. Ternary Oxidized Carbon Nanohorns/TiO2/PVP Nanohybrid as Sensitive Layer for Chemoresistive Humidity Sensor. Chemistry Proceedings. 2021; 5(1):12. https://doi.org/10.3390/CSAC2021-10616
Chicago/Turabian StyleSerban, Bogdan-Catalin, Octavian Buiu, Marius Bumbac, Roxana Marinescu, Niculae Dumbravescu, Viorel Avramescu, Cornel Cobianu, Cristina Mihaela Nicolescu, Mihai Brezeanu, Cristiana Radulescu, and et al. 2021. "Ternary Oxidized Carbon Nanohorns/TiO2/PVP Nanohybrid as Sensitive Layer for Chemoresistive Humidity Sensor" Chemistry Proceedings 5, no. 1: 12. https://doi.org/10.3390/CSAC2021-10616
APA StyleSerban, B.-C., Buiu, O., Bumbac, M., Marinescu, R., Dumbravescu, N., Avramescu, V., Cobianu, C., Nicolescu, C. M., Brezeanu, M., Radulescu, C., & Comanescu, F. (2021). Ternary Oxidized Carbon Nanohorns/TiO2/PVP Nanohybrid as Sensitive Layer for Chemoresistive Humidity Sensor. Chemistry Proceedings, 5(1), 12. https://doi.org/10.3390/CSAC2021-10616









