AlCl3-Catalyzed Synthesis of Zirconacyclopentadienes from Alkynes, Cp2ZrCl2 and Mg †
Abstract
:1. Introduction
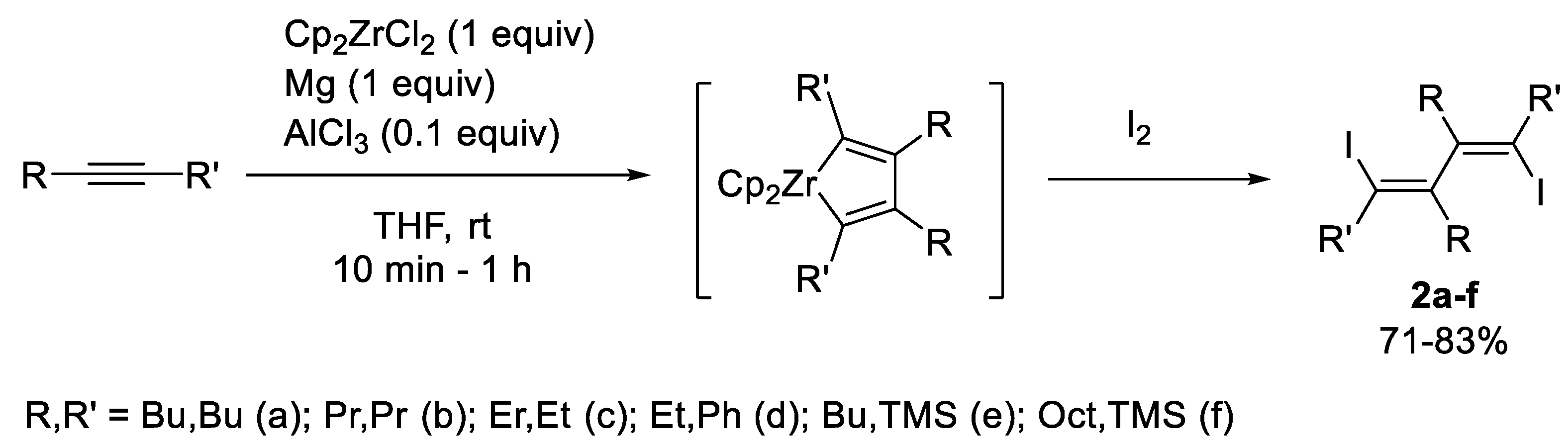
2. Results and Discussion
3. Experimental Part
(5Z,7Z)-6,7-Dibutyl-5,8-Diiodododeca-5,7-Diene (2a)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Negishi, E.; Holmes, S.J.; Tour, J.M.; Miller, J.A.; Cederbaum, F.E.; Swanson, D.R.; Takahashi, T. Novel Bicyclization of Enynes and Diynes Promoted by Zirconocene Derivatives and Conversion of Zirconabicycles into Bicyclic Enones via Carbonylation. J. Am. Chem. Soc. 1989, 111, 3336–3346. [Google Scholar] [CrossRef]
- Negishi, E.; Cederbaum, F.E.; Takahashi, T. Reaction of Zirconocene Dichloride with Alkyllithiums or Alkyl Grignard Reagents as a Convenient Method for Generating a “Zirconocene” Equivalant and Its Use in Zirconium-Promoted Cyclization of Alkenes, Alkynes, Dienes, Enynes, and Diynes. Tetrahedron Lett. 1986, 27, 2829–2832. [Google Scholar] [CrossRef]
- Thanedar, S.; Farona, M.F. A One-Step Synthesis of Bis(H5-Cyclopentadienyl)Zirconacyclopentadiene Compounds. J. Organomet. Chem. 1982, 235, 65–68. [Google Scholar] [CrossRef]
- Hong, J.H. Synthesis and NMR-Study of the 2,3,4,5-Tetraethylsilole Dianion [SiC 4Et 4] 2-•2[Li] +. Molecules 2011, 16, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
 | ||||
|---|---|---|---|---|
| Entry | Lewis Acid | Equiv. | Time | GC Yield of 1, % |
| 1 | AlCl3 | 0.1 | 10 min | 95 |
| 2 | - | - | 18 h | nd |
| 3 | InCl3 | 0.1 | 5 h | nd |
| 4 | SnCl4 | 0.1 | 5 h | nd |
| 5 | Me3SiCl | 0.1 | 5 h | nd |
| 6 | Me3SiCl | 1 | 1 h | 22 |
| 7 | Me3SiCl | 1 | 5 h | 42 |
| 8 | TiCl4 | 0.1 | 5 h | 90 1 |
| 9 | AlCl3 | 0.1 | 5 h | 41 2 |
| 10 | AlCl3 | 0.1 | 5 h | nd 3 |
| 11 | AlCl3 | 0.1 | 2 h | 92 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadykova, F.T.; Zosim, T.P.; Rashitova, A.R.; Dzhemilev, U.M.; Ramazanov, I.R. AlCl3-Catalyzed Synthesis of Zirconacyclopentadienes from Alkynes, Cp2ZrCl2 and Mg. Chem. Proc. 2021, 3, 98. https://doi.org/10.3390/ecsoc-24-08363
Sadykova FT, Zosim TP, Rashitova AR, Dzhemilev UM, Ramazanov IR. AlCl3-Catalyzed Synthesis of Zirconacyclopentadienes from Alkynes, Cp2ZrCl2 and Mg. Chemistry Proceedings. 2021; 3(1):98. https://doi.org/10.3390/ecsoc-24-08363
Chicago/Turabian StyleSadykova, Firuza T., Tat’yana P. Zosim, Aliya R. Rashitova, Usein M. Dzhemilev, and Ilfir R. Ramazanov. 2021. "AlCl3-Catalyzed Synthesis of Zirconacyclopentadienes from Alkynes, Cp2ZrCl2 and Mg" Chemistry Proceedings 3, no. 1: 98. https://doi.org/10.3390/ecsoc-24-08363
APA StyleSadykova, F. T., Zosim, T. P., Rashitova, A. R., Dzhemilev, U. M., & Ramazanov, I. R. (2021). AlCl3-Catalyzed Synthesis of Zirconacyclopentadienes from Alkynes, Cp2ZrCl2 and Mg. Chemistry Proceedings, 3(1), 98. https://doi.org/10.3390/ecsoc-24-08363







