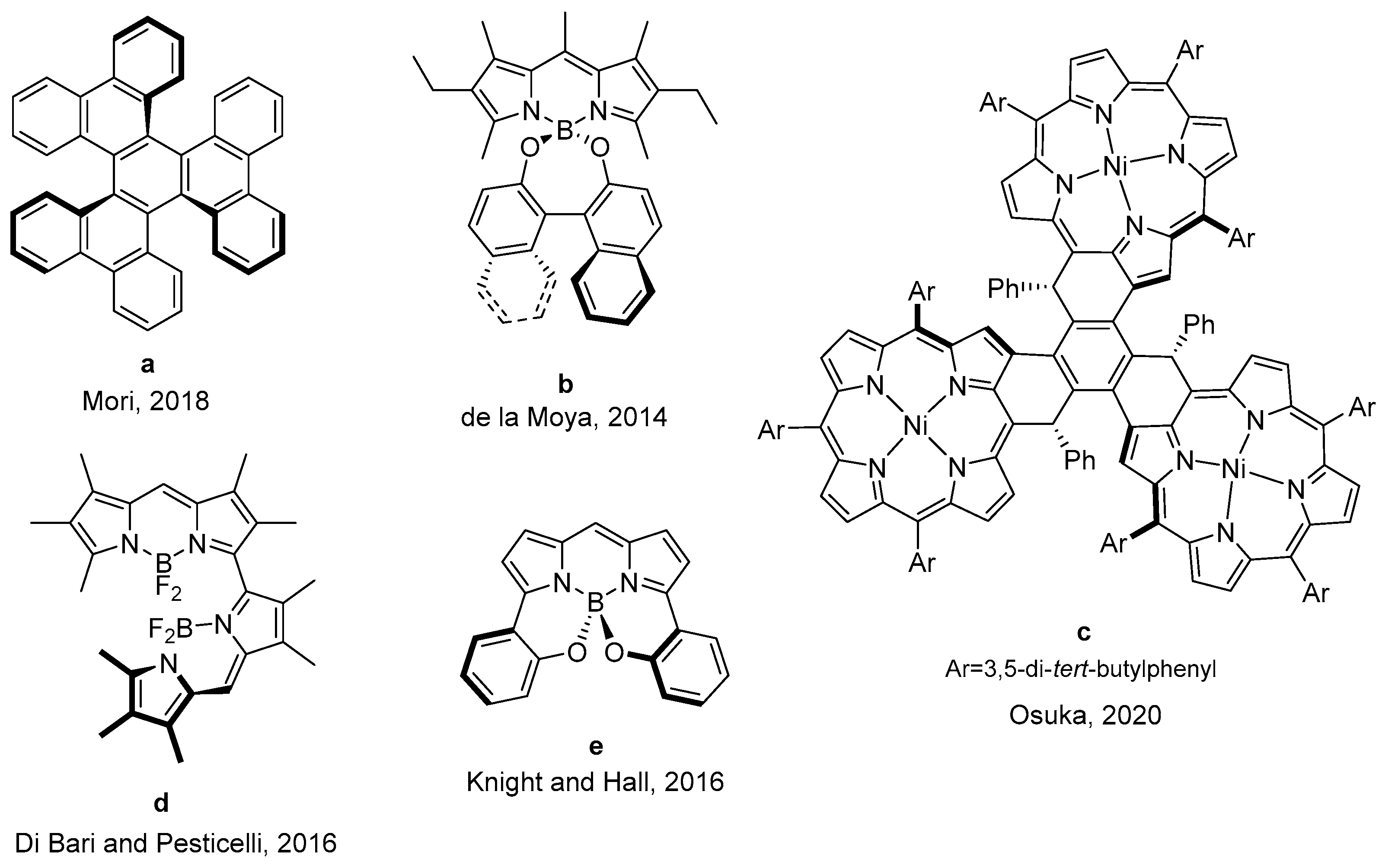
Insight into the Influence of the Chiral Molecular Symmetry on the Chiroptics of Fluorescent BINOL-Based Boron Chelates †
Abstract
:1. Introduction
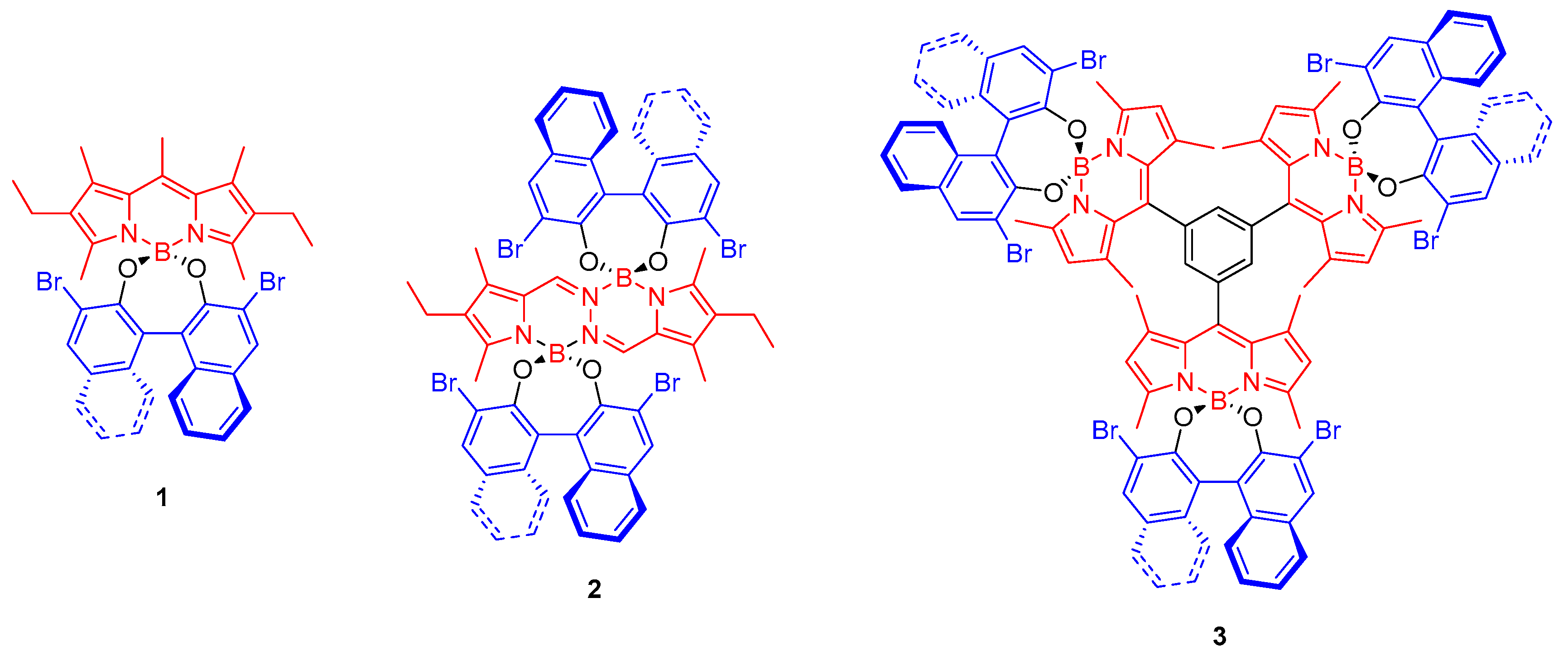
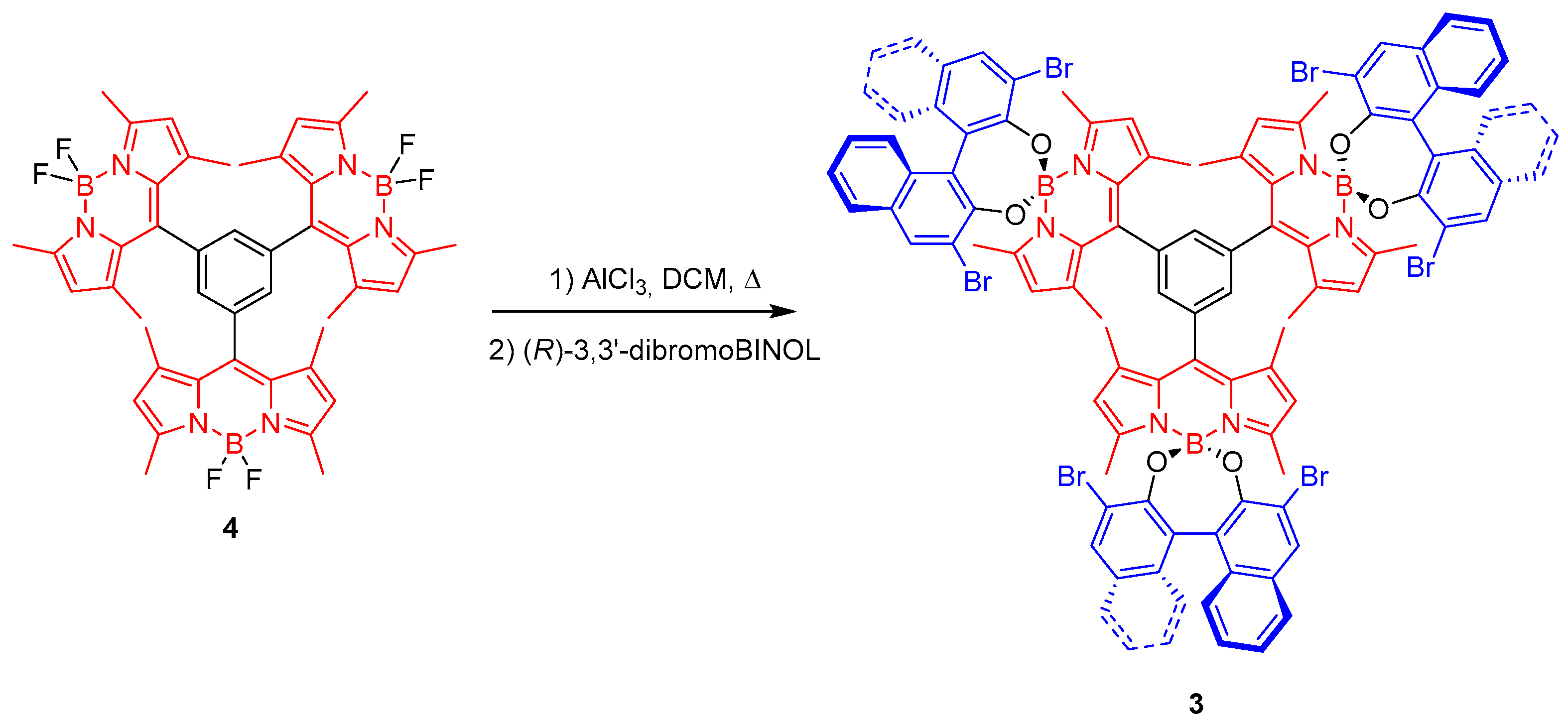
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis
4.2. Spectroscopic Measurements and Quantum Mechanic Calculations
4.3. CD and CPL Measurements
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, T.; Han, J.; Duan, P.; Liu, M. New Perspectives to Trigger and Modulate Circularly Polarized Luminescence of Complex and Aggregated Systems: Energy Transfer, Photon Upconversion, Charge Transfer, and Organic Radical. Acc. Chem. Res. 2020, 53, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Nakashima, T.; Kawai, T. Circularly Polarized Luminescence in Chiral Molecules and Supramolecular Assemblies. J. Phys. Chem. Lett. 2015, 6, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Longhi, G.; Castiglioni, E.; Koshoubu, J.; Mazzeo, G.; Abbate, S. Circularly Polarized Luminescence: A Review of Experimental and Theoretical Aspects. Chirality 2016, 28, 696–707. [Google Scholar] [CrossRef]
- Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M. Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application. Adv. Mater. 2019, 1900110. [Google Scholar] [CrossRef]
- Nagata, Y.; Mori, T. Irreverent Nature of Dissymmetry Factor and Quantum Yield in Circularly Polarized Luminescence of Small Organic Molecules. Front. Chem. 2020, 8, 448. [Google Scholar] [CrossRef]
- Hall, M.J.; de la Moya, S. BODIPY Based Emitters of Circularly Polarized Luminescence. In Circularly Polarized Luminescence of Isolated Small Organic Molecules; Mori, T., Ed.; Springer: Singapore, 2020; pp. 117–149. [Google Scholar]
- Sánchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; de la Moya, S. Circularly Polarized Luminescence from Simple Organic Molecules. Chem. Eur. J. 2015, 21, 13488–13500. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Inoue, Y.; Mori, T. Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation between Excitation and Emission Dissymmetry Factors. ChemPhotoChem 2018, 2, 386–402. [Google Scholar] [CrossRef]
- Ma, J.; Peng, Q.; Zhao, C. Circularly Polarized Luminescence Switching in Small Organic Molecules. Chem. Eur. J. 2019, 25, 15441–15454. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kato, Y.; Fujiki, M.; Inoue, Y.; Mori, T. Combined Experimental and Theoretical Study on Circular Dichroism and Circularly Polarized Luminescence of Configurationally Robust D3-Symmetric Triple Pentahelicene. J. Phys. Chem. A 2018, 122, 7378–7384. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carnerero, E.M.; Moreno, F.; Maroto, B.L.; Agarrabeitia, A.R.; Ortiz, M.J.; Vo, B.G.; Muller, G.; de la Moya, S. Circularly Polarized Luminescence by Visible-Light Absorption in a Chiral O-BODIPY Dye: Unprecedented Design of CPL Organic Molecules from Achiral Chromophores. J. Am. Chem. Soc. 2014, 136, 3346–3349. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Osuka, A. Propeller-Shaped Semi-fused Porphyrin Trimers: Molecular-Symmetry-Dependent Chiroptical Response. Chem. Eur. J. 2020, 26, 10217–10221. [Google Scholar] [CrossRef] [PubMed]
- Zinna, F.; Bruhn, T.; Guido, C.A.; Ahrens, J.; Broering, M.; Di Bari, L.; Pescitelli, G. Circularly polarized luminescence from axially chiral BODIPY DYEmers: An experimental and computational study. Chem. Eur. J. 2016, 22, 16089–16098. [Google Scholar] [CrossRef] [PubMed]
- Alnoman, R.B.; Rihn, S.; O’Connor, D.C.; Black, F.A.; Costello, B.; Waddell, P.G.; Clegg, W.; Peacock, R.D.; Herrebout, W.; Knight, J.G.; et al. Circularly Polarized Luminescence from Helically Chiral N,N,O,O-Boron-Chelated Dipyrromethenes. Chem. Eur. J. 2016, 22, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ikenosako, M.; Kato, Y. Symmetry-based rational design for boosting chiroptical responses. Commun. Chem. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Jiménez, J.; Cerdán, L.; Moreno, F.; Maroto, B.L.; García-Moreno, I.; Lunkley, J.L.; Muller, G.; de la Moya, S. Chiral Organic Dyes Endowed with Circularly Polarized Laser Emission. J. Phys. Chem. C 2017, 121, 5287–5292. [Google Scholar] [CrossRef] [PubMed]
- Sola-Llano, R.; Jiménez, J.; Avellanal-Zaballa, E.; Johnson, M.; Cabreros, T.A.; Moreno, F.; Maroto, B.L.; Muller, G.; Bañuelos, J.; Cerdán, L.; et al. BOPHYs versus BODIPYs: A comparison of their performance as effective multi-function organic dyes. Dyes Pigments 2019, 170, 107662. [Google Scholar] [CrossRef] [PubMed]
- gabs is the Kuhn dissymmetry ratio, defined by gabs(λ) = 2Δε/ε = 2(εL − εR)/(εL + εR), where εL and εR refer, respectively, to the molar absorptivity of left and right circularly polarized light. (See ref. 7).
- Liu, Y.; Song, N.; Chen, L.; Xie, Z. Triple-BODIPY organic nanoparticles with particular fluorescence emission. Dyes Pigments 2017, 147, 241–245. [Google Scholar] [CrossRef]
- Kellog, M.; Akil, A.; Ravinson, D.S.M.; Estergren, L.; Bradforth, S.E.; Thompson, M.E. Symmetry breaking charge transfer as a means to study electron transfer with no driving force. Faraday Discuss. 2019, 216, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Brunet, E.; Jiménez, L.; de Victoria-Rodríguez, M.; Luu, V.; Muller, G.; Juanes, O.; Rodríguez-Ubis, J.C. The use of lanthanide luminescence as a reporter in the solid state: Desymmetrization of the prochiral layers of γ-zirconium phosphate/phosphonate and circularly polarized luminescence. Microporous Mesoporous Mater. 2013, 169, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, H.P.J.M.; Moraal, P.F.; Timper, J.M.; Riehl, J.P. Optical Artifacts in Circularly Polarized Luminescence Spectroscopy. Appl. Spectrosc. 1985, 39, 818–821. [Google Scholar] [CrossRef]
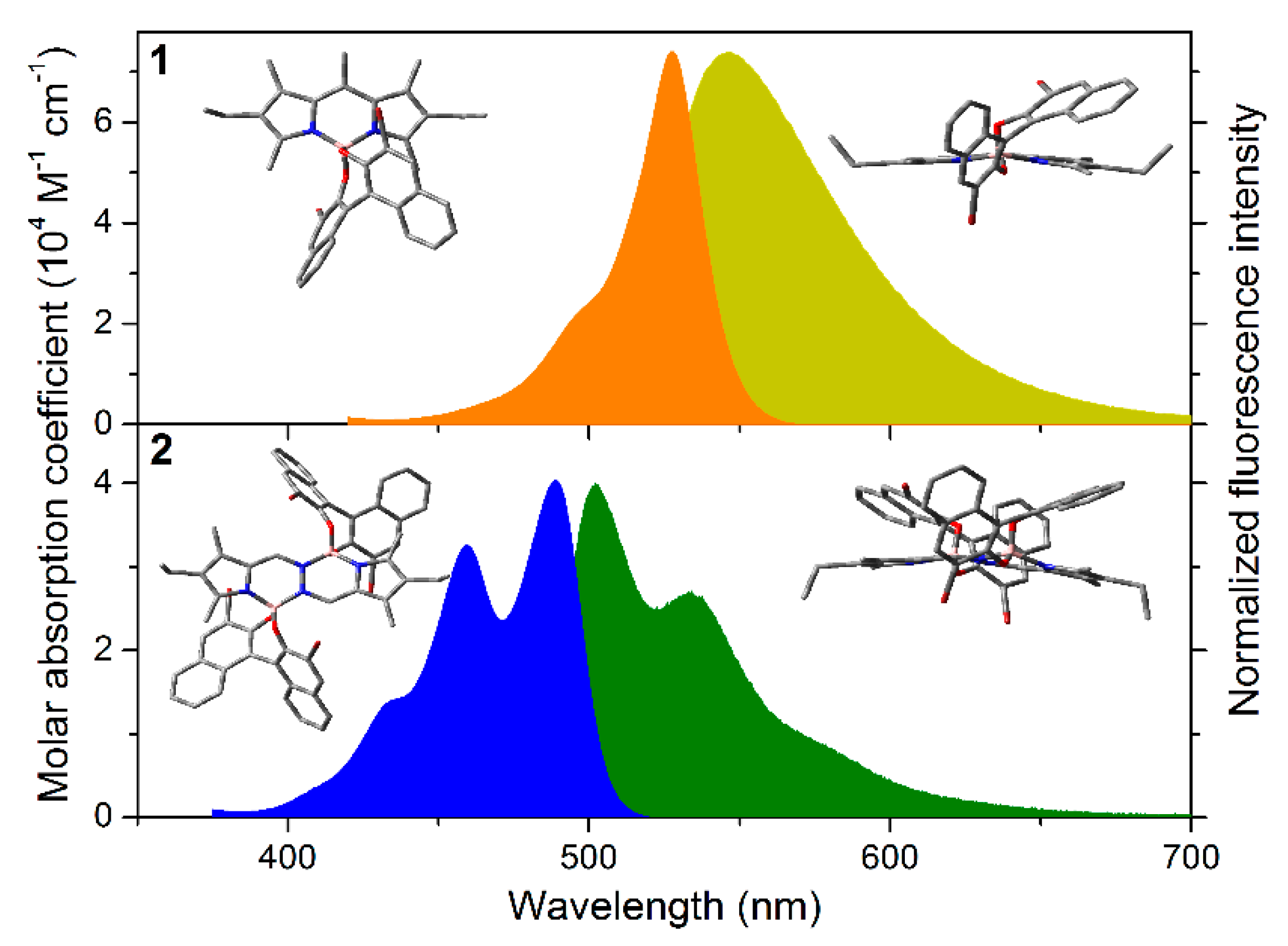
| Dye | gabs·103 | glum·103 |
|---|---|---|
| 1 | −0.9 | −0.6 |
| 2 | −0.4 | −1.4 |
| 3 | −1.4 | −2.0 |
| Dye | λab (nm) | εmax (104 M−1 cm−1) | λfl (nm) | φ | Τ (ns) |
|---|---|---|---|---|---|
| 1 | 527.0 | 7.4 | 547.0 | 0.69 | 6.56 |
| 2 | 486.0 | 4.0 | 501.0 | 0.48 | 2.00 |
| 3 | 519.0 | 10.5 | 551.0 | 0.04 | 0.37 (93%)/2.31 (7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, J.; Avellanal-Zaballa, E.; Serrano, S.; Torres, A.R.; Agarrabeitia, A.R.; Moreno, F.; Muller, G.; Bañuelos, J.; Maroto, B.L.; Moya, S.d.l. Insight into the Influence of the Chiral Molecular Symmetry on the Chiroptics of Fluorescent BINOL-Based Boron Chelates. Chem. Proc. 2021, 3, 76. https://doi.org/10.3390/ecsoc-24-08308
Jiménez J, Avellanal-Zaballa E, Serrano S, Torres AR, Agarrabeitia AR, Moreno F, Muller G, Bañuelos J, Maroto BL, Moya Sdl. Insight into the Influence of the Chiral Molecular Symmetry on the Chiroptics of Fluorescent BINOL-Based Boron Chelates. Chemistry Proceedings. 2021; 3(1):76. https://doi.org/10.3390/ecsoc-24-08308
Chicago/Turabian StyleJiménez, Josué, Edurne Avellanal-Zaballa, Sergio Serrano, Alexis R. Torres, Antonia R. Agarrabeitia, Florencio Moreno, Gilles Muller, Jorge Bañuelos, Beatriz L. Maroto, and Santiago de la Moya. 2021. "Insight into the Influence of the Chiral Molecular Symmetry on the Chiroptics of Fluorescent BINOL-Based Boron Chelates" Chemistry Proceedings 3, no. 1: 76. https://doi.org/10.3390/ecsoc-24-08308
APA StyleJiménez, J., Avellanal-Zaballa, E., Serrano, S., Torres, A. R., Agarrabeitia, A. R., Moreno, F., Muller, G., Bañuelos, J., Maroto, B. L., & Moya, S. d. l. (2021). Insight into the Influence of the Chiral Molecular Symmetry on the Chiroptics of Fluorescent BINOL-Based Boron Chelates. Chemistry Proceedings, 3(1), 76. https://doi.org/10.3390/ecsoc-24-08308