Evaluation of Fluorescent Staining Capacity of Two New Nile Blue Analogues †
Abstract
:1. Introduction
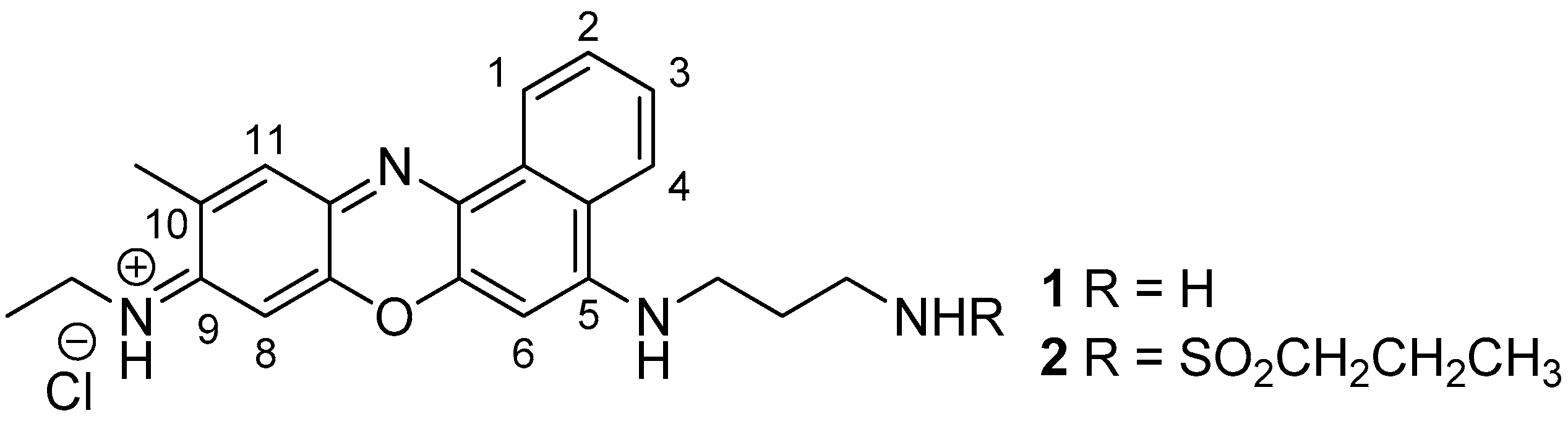
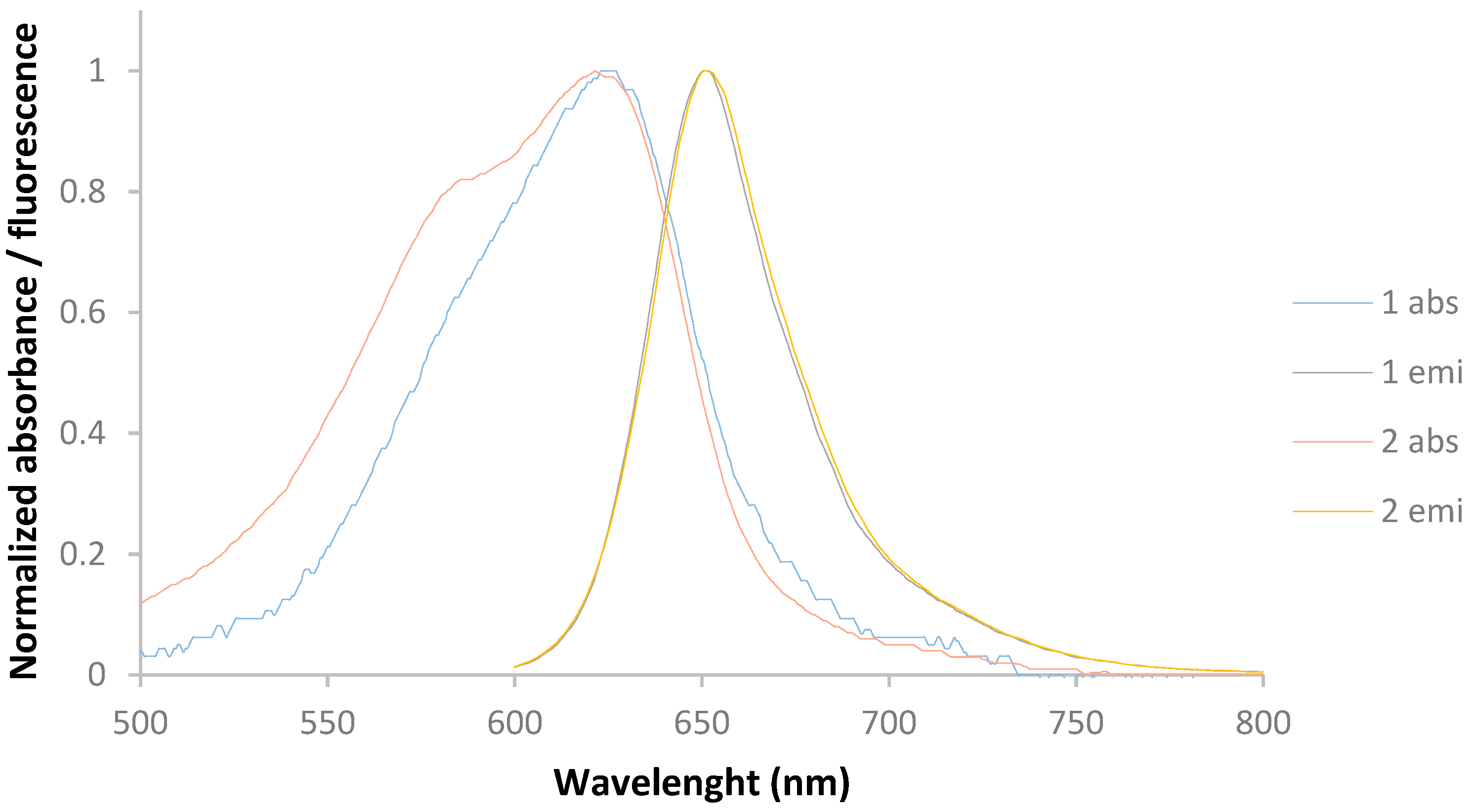
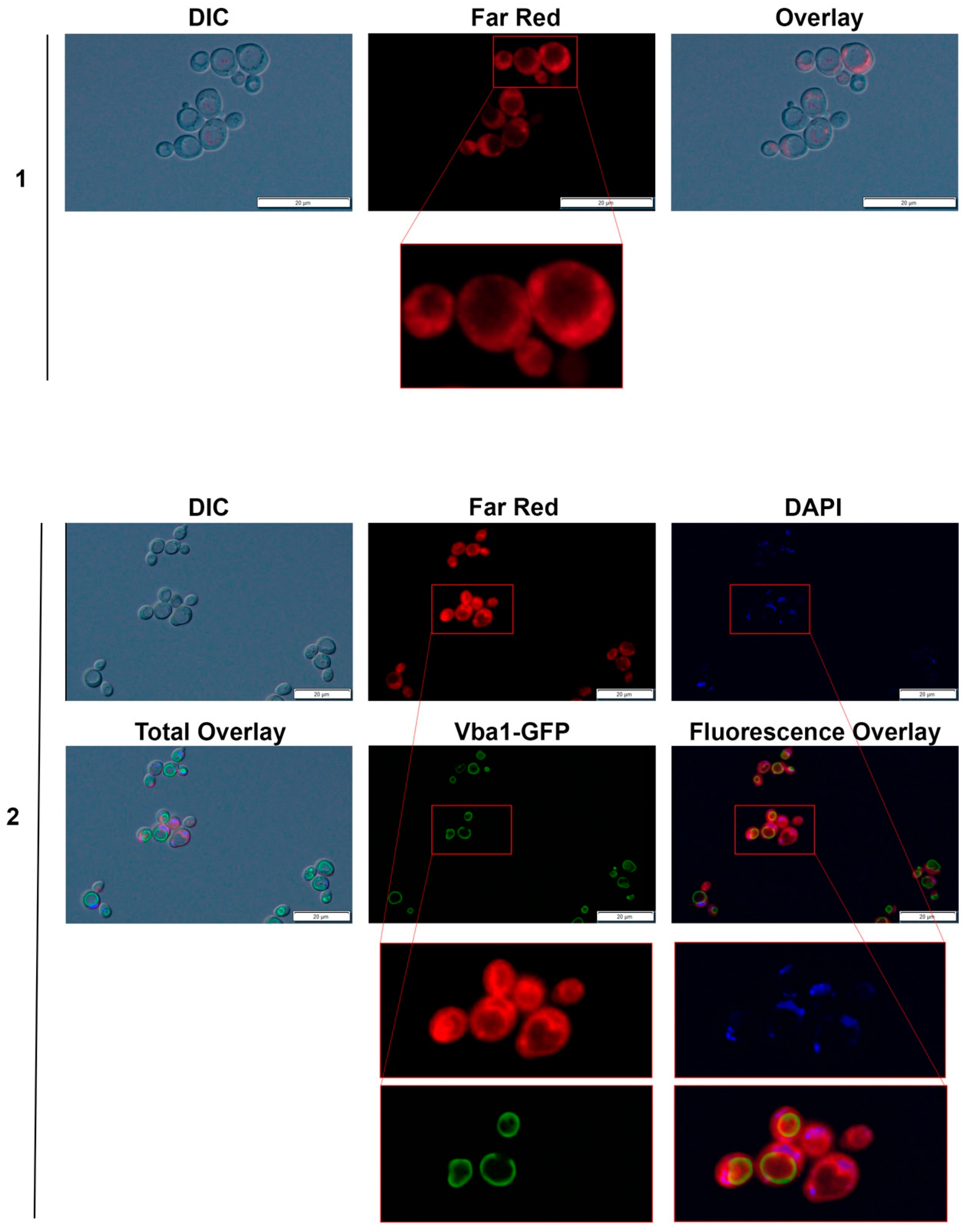
2. Results and Discussion
3. Experimental
3.1. Typical Procedure for the Preparation of benzo[a]phenoxazinium Chlorides (Illustrated for 2)
3.2. Procedure for Fluorescence Staining
4. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, M.S.T. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.H.J.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Moura, J.C.V.P. Synthesis and spectral properties of long-wavelength fluorescent dyes. J. Photochem. Photobiol. A Chem. 2007, 185, 220–230. [Google Scholar] [CrossRef]
- Jose, J.; Burgess, K. Syntheses and properties of water-soluble Nile Red derivatives. J. Org. Chem. 2006, 71, 7835–7839. [Google Scholar] [CrossRef] [PubMed]
- Firmino, A.D.G.; Gonçalves, M.S.T. Bifunctionalised long-wavelength fluorescent probes for biological applications. Tetrahedron Lett. 2012, 53, 4946–4950. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Ueno, Y.; Burgess, K. Water-soluble nile blue derivatives: Syntheses and photophysical properties. Chem. Eur. J. 2009, 15, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel long alkyl side chain benzo[a]phenoxazinium chlorides: Synthesis, photophysical behaviour and DNA interaction. Tetrahedron 2009, 65, 10441–10452. [Google Scholar] [CrossRef]
- Salomi, B.S.B.; Mitra, C.K.; Gorton, L. Electrochemical and spectrophotometric studies on dyes and proteins labelled with dyes. Synth. Met. 2005, 155, 426–429. [Google Scholar] [CrossRef]
- Rama, R.B.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel Nile Blue derivatives as fluorescent probes for DNA. Dyes Pigments 2013, 99, 220–227. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of short and long-wavelength functionalised probes: Amino acids’ labelling and photophysical studies. Tetrahedron 2007, 50, 12405–12418. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Rama Raju, B.; Cerqueira, N.M.F.S.A.; Sousa, M.J.; Gonçalves, M.S.T. Benzo[a]phenoxazinium chlorides: Synthesis, antifungal activity, in silico studies and evaluation as fluorescent probes. Bioorg. Chem. 2020, 98, 103730. [Google Scholar] [CrossRef] [PubMed]

| Compound | 1 | 2 |
|---|---|---|
| Log P | 0.96 | 2.20 |
| Acidified ethanol | ||
| λabs (nm) | 627 | 627 |
| Log ε (M−1 cm−1) | 5.17 | 4.60 |
| λemi (nm) | 644 | 643 |
| ΦF | 0.58 | 0.58 |
| ∆λ (nm) | 17 | 16 |
| pH 7.4 | ||
| λabs (nm) | 627 | 621 |
| Log ε (M−1 cm−1) | 3.90 | 4.40 |
| λemi (nm) | 651 | 649 |
| ΦF | 0.24 | 0.20 |
| ∆λ (nm) | 24 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.C.C.; Sousa, R.P.C.L.; Sousa, M.J.M.F.; Gonçalves, M.S.T. Evaluation of Fluorescent Staining Capacity of Two New Nile Blue Analogues. Chem. Proc. 2021, 3, 63. https://doi.org/10.3390/ecsoc-24-08383
Ferreira JCC, Sousa RPCL, Sousa MJMF, Gonçalves MST. Evaluation of Fluorescent Staining Capacity of Two New Nile Blue Analogues. Chemistry Proceedings. 2021; 3(1):63. https://doi.org/10.3390/ecsoc-24-08383
Chicago/Turabian StyleFerreira, João C. C., Rui P. C. L. Sousa, Maria João M. F. Sousa, and M. Sameiro T. Gonçalves. 2021. "Evaluation of Fluorescent Staining Capacity of Two New Nile Blue Analogues" Chemistry Proceedings 3, no. 1: 63. https://doi.org/10.3390/ecsoc-24-08383
APA StyleFerreira, J. C. C., Sousa, R. P. C. L., Sousa, M. J. M. F., & Gonçalves, M. S. T. (2021). Evaluation of Fluorescent Staining Capacity of Two New Nile Blue Analogues. Chemistry Proceedings, 3(1), 63. https://doi.org/10.3390/ecsoc-24-08383







