Synthesis of Triphenylamine-Imidazo[1,2-a]pyridine via Groebke–Blackburn–Bienaymé Reaction †
Abstract
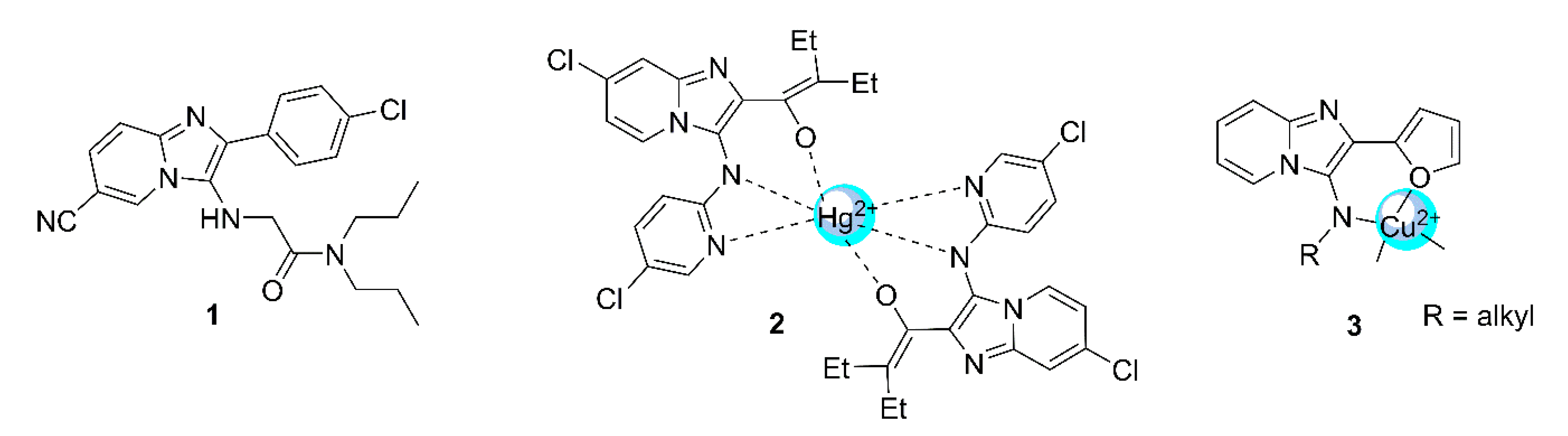
:1. Introduction
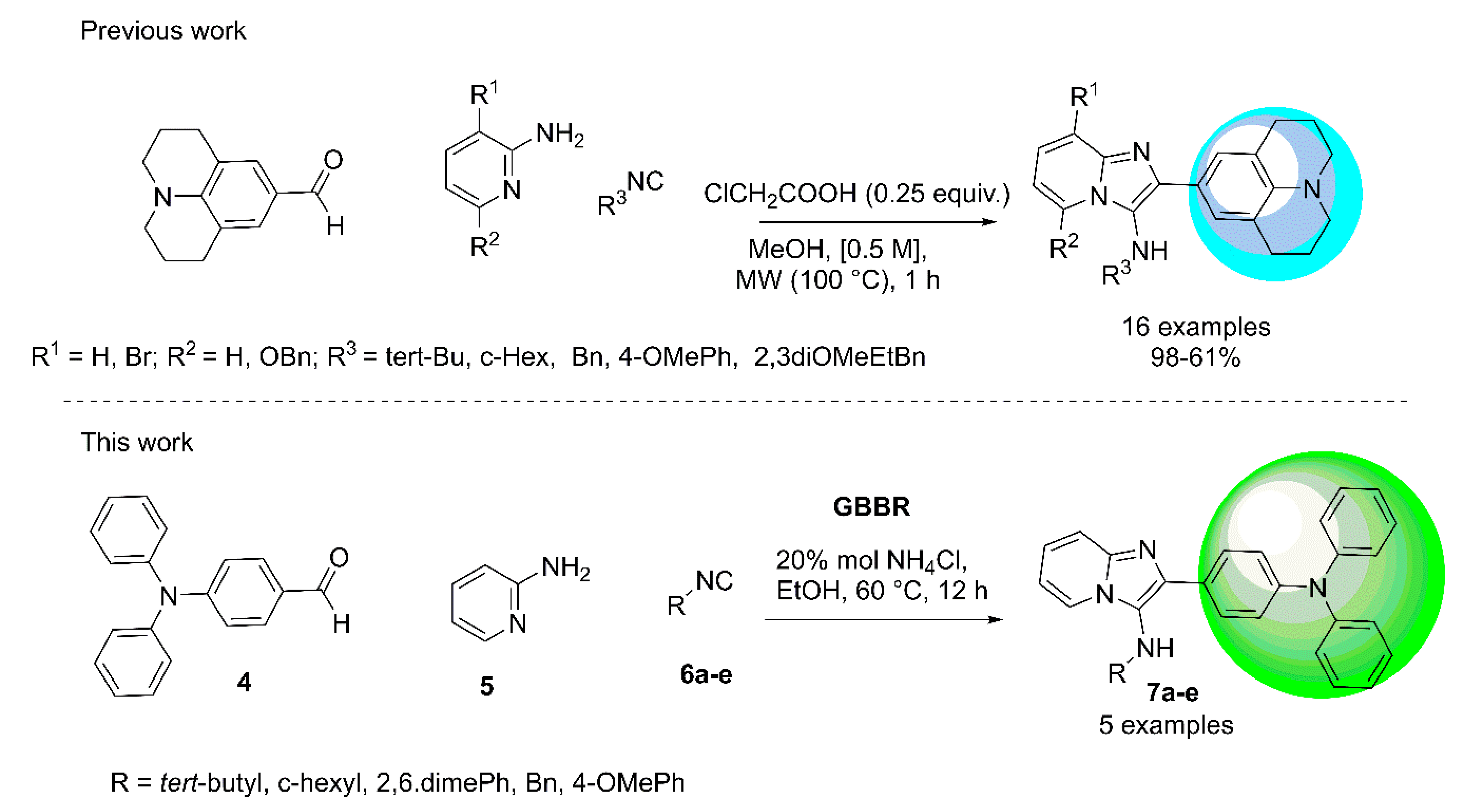
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. Synthesis and Characterization of the Imidazo[1,2-a]pyridine-Triphenylamines (7a–e)
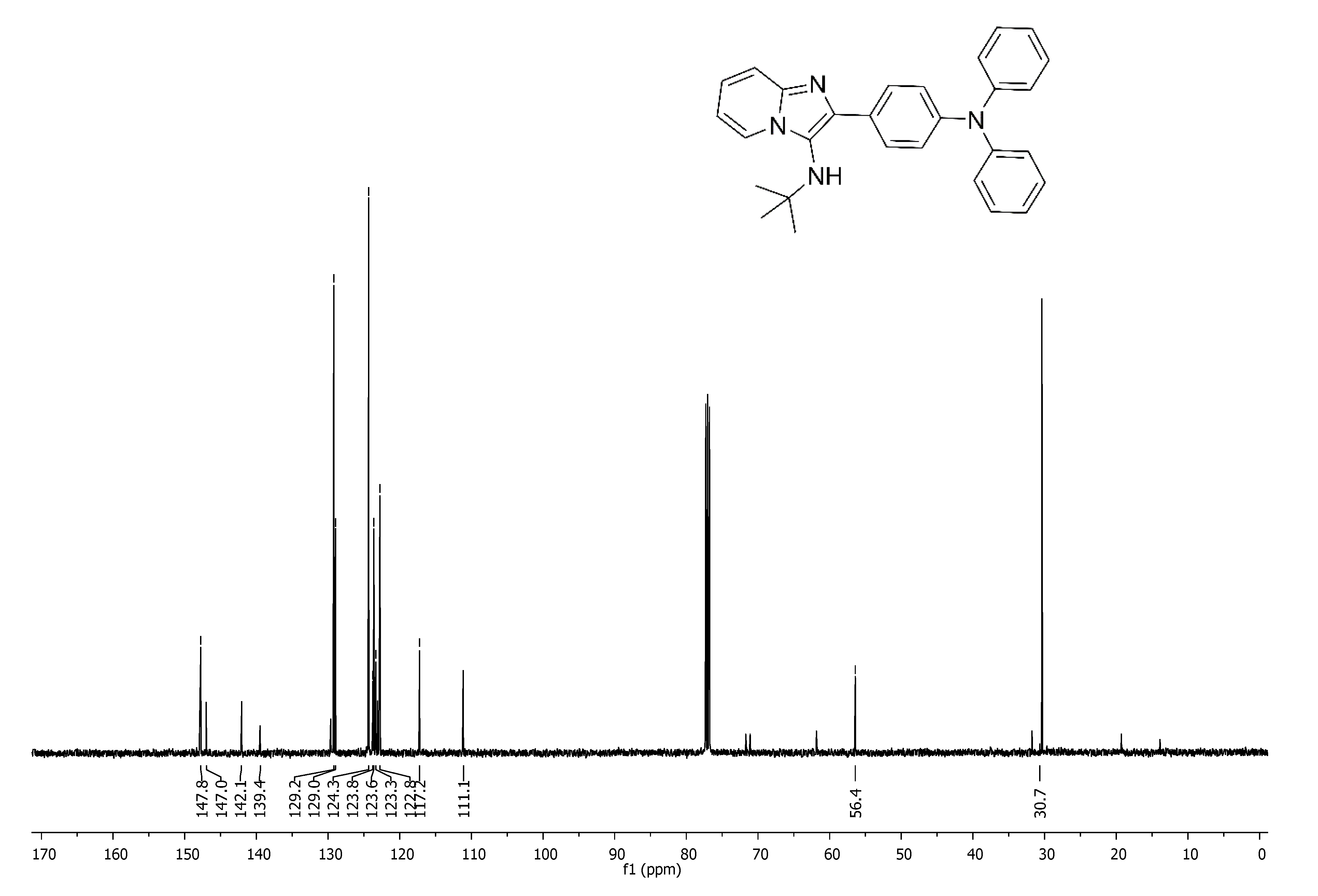
3.2.1. N-(tert-Butyl)-2-(4-(diphenylamino)phenyl)imidazo[1,2-a]pyridine-3-amine (7a)
3.2.2. N-Cyclohexyl-2-(4-(diphenylamino)phenyl)imidazo[1,2-a]pyridin-3-amine (7b)
3.2.3. N-(2,6-Dimethylphenyl)-2-(4-(diphenylamino)phenyl)imidazo[1,2-a]pyridin-3-amine (7c)
3.2.4. N-Benzyl-2-(4-(diphenylamino)phenyl)imidazo[1,2-a]pyridin-3-amine (7d)
3.2.5. 2-(4-(Diphenylamino)phenyl)-N-(4-methoxyphenyl)imidazo[1,2-a]pyridin-3-amine (7e)
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Couty, F.; Evano, G. Comprehensive Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 11, p. 409. [Google Scholar]
- Song, G.; Zhang, Y.; Li, X. Rhodium and Iridium Complexes of Abnormal N-Heterocyclic Carbenes Derived from Imidazo[1,2-a]pyridine. Organometallics 2008, 27, 1936–1943. [Google Scholar] [CrossRef]
- John, A.; Shaikh, M.M.; Ghosh, P. Palladium complexes of abnormal N-heterocyclic carbenes as precatalysts for the much preferred Cu-free and amine-free Sonogashira coupling in air in a mixed-aqueous medium. Dalton Trans. 2009, 2009, 10581–10591. [Google Scholar] [CrossRef] [PubMed]
- Enguehard-Gueiffier, C.; Gueiffier, A. Recent Progress in the Pharmacology of Imidazo[1,2-a]pyridines Mini Rev. Med. Chem. 2007, 7, 888–899. [Google Scholar] [CrossRef]
- Burchak, O.N.; Mugherli, L.; Ostuni, M.; Lacapère, J.J.; Balakirev, M.Y. Combinatorial Discovery of Fluorescent Pharmacophores by Multicomponent Reactions in Droplet Arrays. J. Am. Chem. Soc. 2011, 133, 10058–10061. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Pang, G.-X.; Yan, C.-X.; Shi, G.-F.; Cheng, Y. Reaction of β-Lactam Carbenes with 2-Pyridyl Isonitriles: A One-Pot Synthesis of 2-Carbonyl-3-(pyridylamino)imidazo[1,2-a]pyridines Useful as Fluorescent Probes for Mercury Ion. J. Org. Chem. 2011, 76, 7458–7465. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, L.K.; Kumar, M.; Bhatt, P.; Sharma, A.; Asif, M.; Gupta, V.K. An easily accessible optical chemosensor for Cu2+ based on novel imidazoazine framework, its performance characteristics and potential applications. Sens. Actuators B. Chem. 2017, 240, 365–375. [Google Scholar] [CrossRef]
- Kwon, O.; Barlow, S.; Odom, S.A.; Beverina, L.; Thompson, N.J.; Zojer, E.; Bredas, J.L.; Marder, S.R. Aromatic Amines: A Comparison of Electron-Donor Strengths. J. Phys. Chem. A 2005, 109, 9346–9352. [Google Scholar] [CrossRef] [PubMed]
- Roquet, S.; Cravino, A.; Leriche, P.; Alévêque, O.; Frère, P.; Roncali, J. Triphenylamine−Thienylenevinylene Hybrid Systems with Internal Charge Transfer as Donor Materials for Heterojunction Solar Cells. J. Am. Chem. Soc. 2006, 128, 3459–3466. [Google Scholar] [CrossRef]
- Ning, Z.J.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials Chem. Comm. 2009, 37, 5483–5495. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Tsai, C.-H.; Wong, K.-T.; Huang, T.-W.; Hsieh, L.; Liu, S.H.; Lin, H.-W.; Wu, C.-C.; Chou, S.-H.; Chen, S.-H.; et al. Organic Dyes Containing Coplanar Diphenyl-Substituted Dithienosilole Core for Efficient Dye-Sensitized Solar Cells. J. Org. Chem. 2010, 75, 4778–4785. [Google Scholar] [CrossRef]
- Levi, L.; Muller, T.J.J. Multicomponent syntheses of functional chromophores. Chem. Soc. Rev. 2016, 45, 2825–2846. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Pericherla, K.; Kaswan, P.; Pandey, K.; Kumar, A. Recent Developments in the Synthesis of Imidazo[1,2-a]pyridines. Synthesis 2015, 47, 887–912. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Santra, S.; Monir, K.; Hajra, A. Synthesis of imidazo[1,2-a]pyridines: A decade update. Chem. Commun. 2015, 51, 1555–1575. [Google Scholar] [CrossRef] [PubMed]
- Groebke, K.; Weber, L.; Mehlin, F. Synthesis of Imidazo[1,2-a] annulated Pyridines, Pyrazines and Pyrimidines by a Novel Three-Component Condensation. Synlett 1998, 6, 661–663. [Google Scholar] [CrossRef]
- Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Parallel synthesis of 3-aminoimidazo[1,2-a]pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 1998, 39, 3635–3638. [Google Scholar] [CrossRef]
- Bienaymé, H.; Bouzid, K.A. New Heterocyclic Multicomponent Reaction for the Combinatorial Synthesis of Fused 3-Aminoimidazoles. Angew. Chem. 1998, 110, 2349–2352. [Google Scholar] [CrossRef]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebkee-Blackburne-Bienayme reaction: A review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Volkova, Y.; Gevorgyan, V. Synthesis of functionalyzed imidazo[1,2-a]pyridines via domino A3-coupling/cycloisomerization approach. Chem. Heterocycl. Compd. 2017, 53, 409–412. [Google Scholar] [CrossRef]
- Shaaban, S.; Abdel-Wahab, B.F. Groebke-Blackburn-Bienaymé multicomponent reaction: Emerging chemistry for drug discovery. Mol. Divers. 2016, 20, 233–254. [Google Scholar] [CrossRef]
- Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. One-Pot Synthesis of Quinoline-Based Tetracycles by a Tandem Three-Component Reaction. J. Comb. Chem. 2007, 9, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Soleimani, E.; Maleki, A.; Moghimi-Rad, J. A novel class of extended pi-conjugated systems: One-pot synthesis of bis-3-aminoimidazo[1,2-a]pyridines, pyrimidines and pyrazines. Mol. Divers. 2009, 13, 269–274. [Google Scholar] [CrossRef]
- Basavanag, U.M.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Conejo, A.S.; Kurva, M.; Jiménez-Halla, J.O.C.; Velusamy, J.; Ramos-Ortíz, G.; Gámez-Montaño, R. Synthesis of 2-julolidin-imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé reaction and studies of optical properties. New J. Chem. 2017, 41, 3450–3459. [Google Scholar] [CrossRef]
- Claudio-Catalán, M.A.; Pharande, S.G.; Quezada-Soto, A.; Kishore, K.G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis- Heterocycles Synthesis via Groebke-Blackburn-Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo[1,2-a]pyridines via Groebke-Blackburn-Bienayme reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]


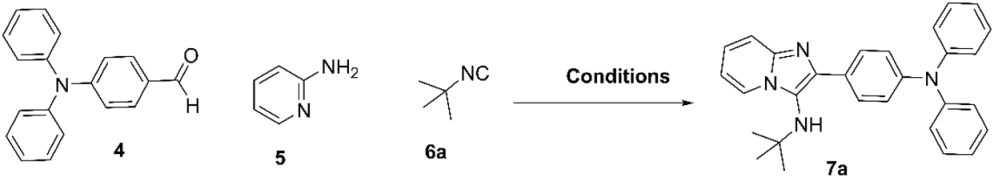 | ||||
|---|---|---|---|---|
| Entry a | Solvent b | Aditive | T (°C) | Yield (%) c |
| 1 | --- | --- | rt | nr |
| 2 | H2O | --- | rt | nd |
| 3 | EtOH | --- | rt | 30 |
| 4 | EtOH | NH4Cl (20% mol) | rt | 45 |
| 5 | EtOH | NH4Cl (20% mol) | 60 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, M.A.R.; Kurva, M.; Gámez-Montaño, R. Synthesis of Triphenylamine-Imidazo[1,2-a]pyridine via Groebke–Blackburn–Bienaymé Reaction. Chem. Proc. 2021, 3, 61. https://doi.org/10.3390/ecsoc-24-08422
Gómez MAR, Kurva M, Gámez-Montaño R. Synthesis of Triphenylamine-Imidazo[1,2-a]pyridine via Groebke–Blackburn–Bienaymé Reaction. Chemistry Proceedings. 2021; 3(1):61. https://doi.org/10.3390/ecsoc-24-08422
Chicago/Turabian StyleGómez, Manuel A. Rentería, Mahanandaiah Kurva, and Rocío Gámez-Montaño. 2021. "Synthesis of Triphenylamine-Imidazo[1,2-a]pyridine via Groebke–Blackburn–Bienaymé Reaction" Chemistry Proceedings 3, no. 1: 61. https://doi.org/10.3390/ecsoc-24-08422
APA StyleGómez, M. A. R., Kurva, M., & Gámez-Montaño, R. (2021). Synthesis of Triphenylamine-Imidazo[1,2-a]pyridine via Groebke–Blackburn–Bienaymé Reaction. Chemistry Proceedings, 3(1), 61. https://doi.org/10.3390/ecsoc-24-08422






