Glucose-Based Molecular Rotors as Fluorescent Inhibitors and Probes of Glycogen Phosphorylase †
Abstract
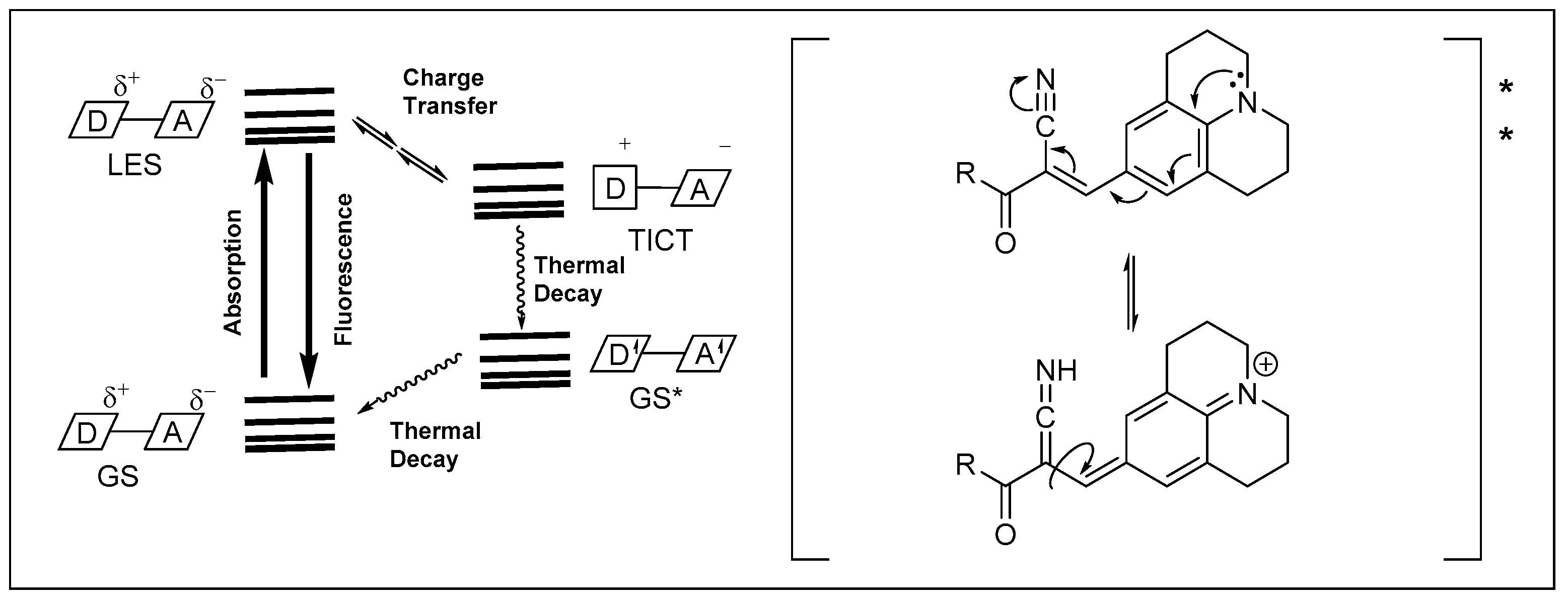
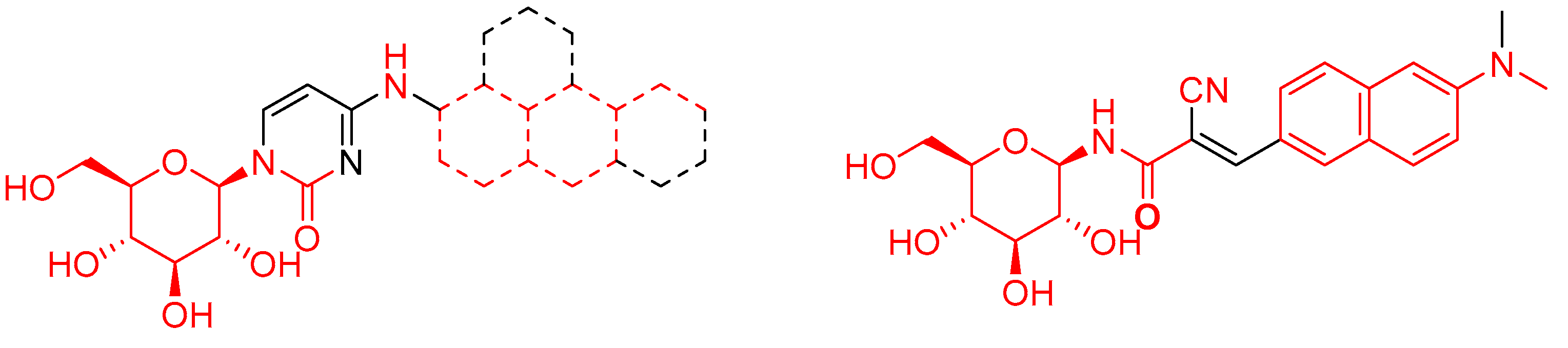
1. Introduction
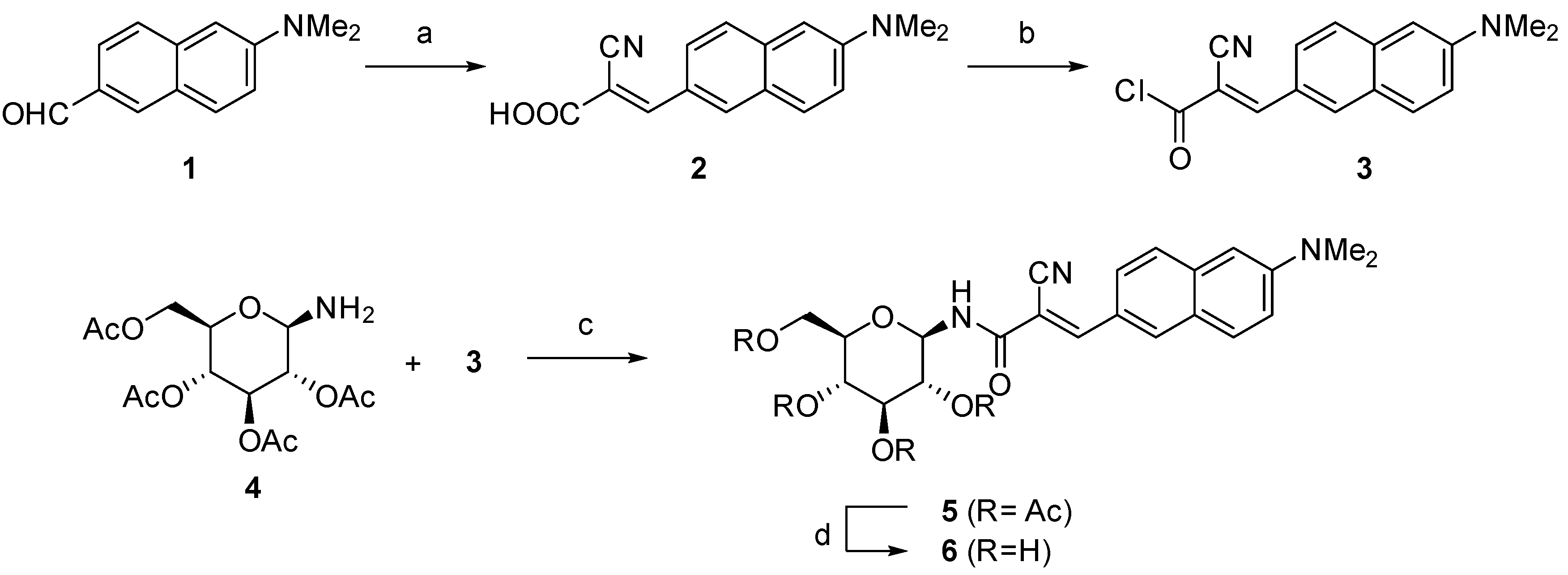
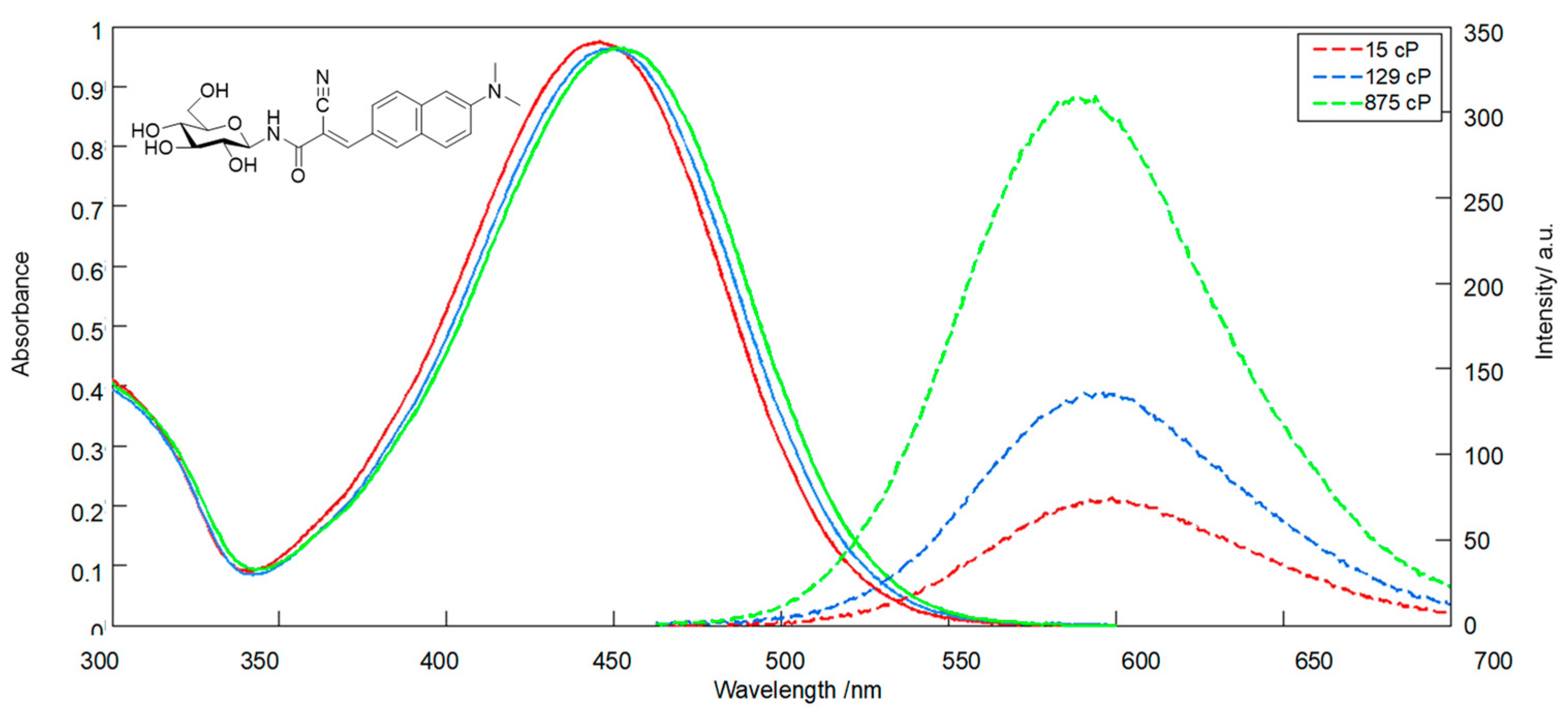
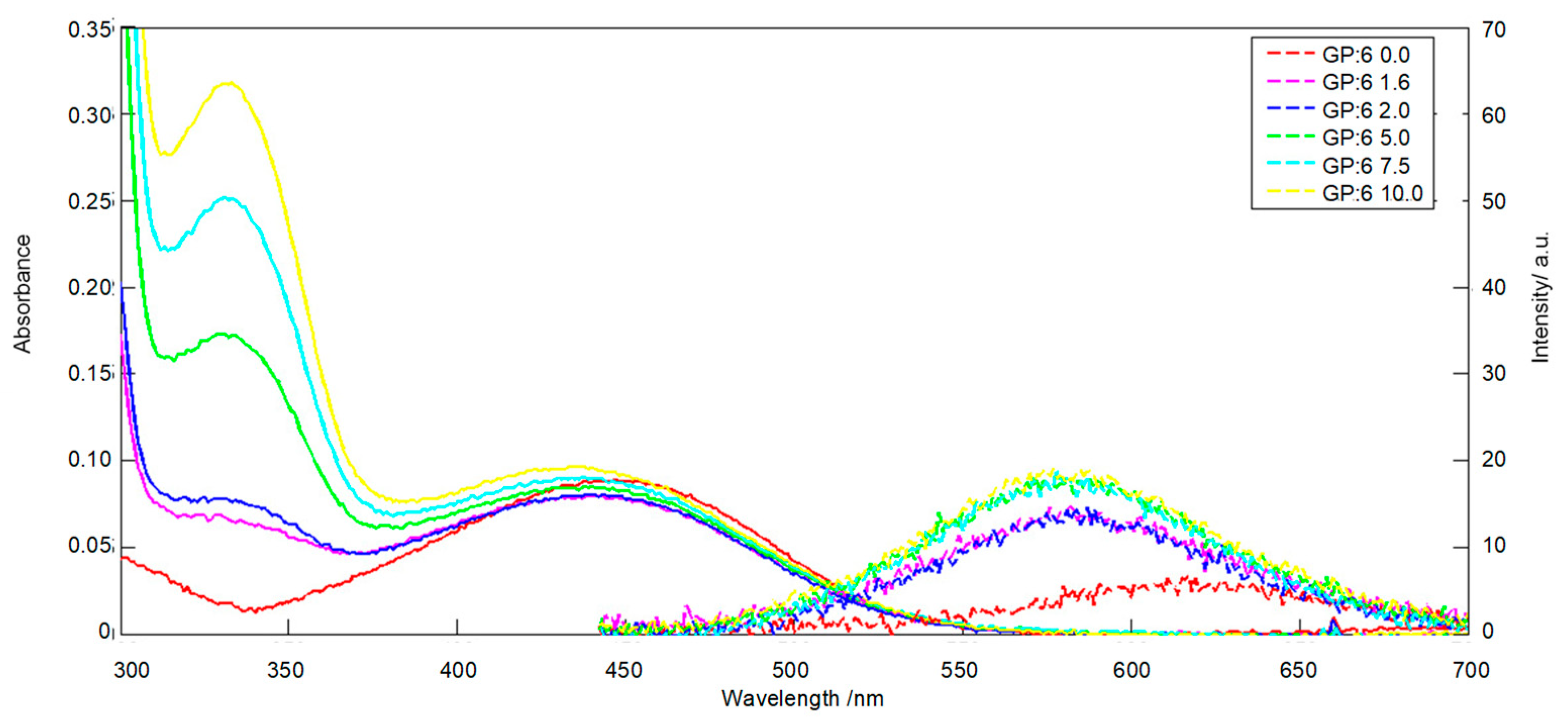
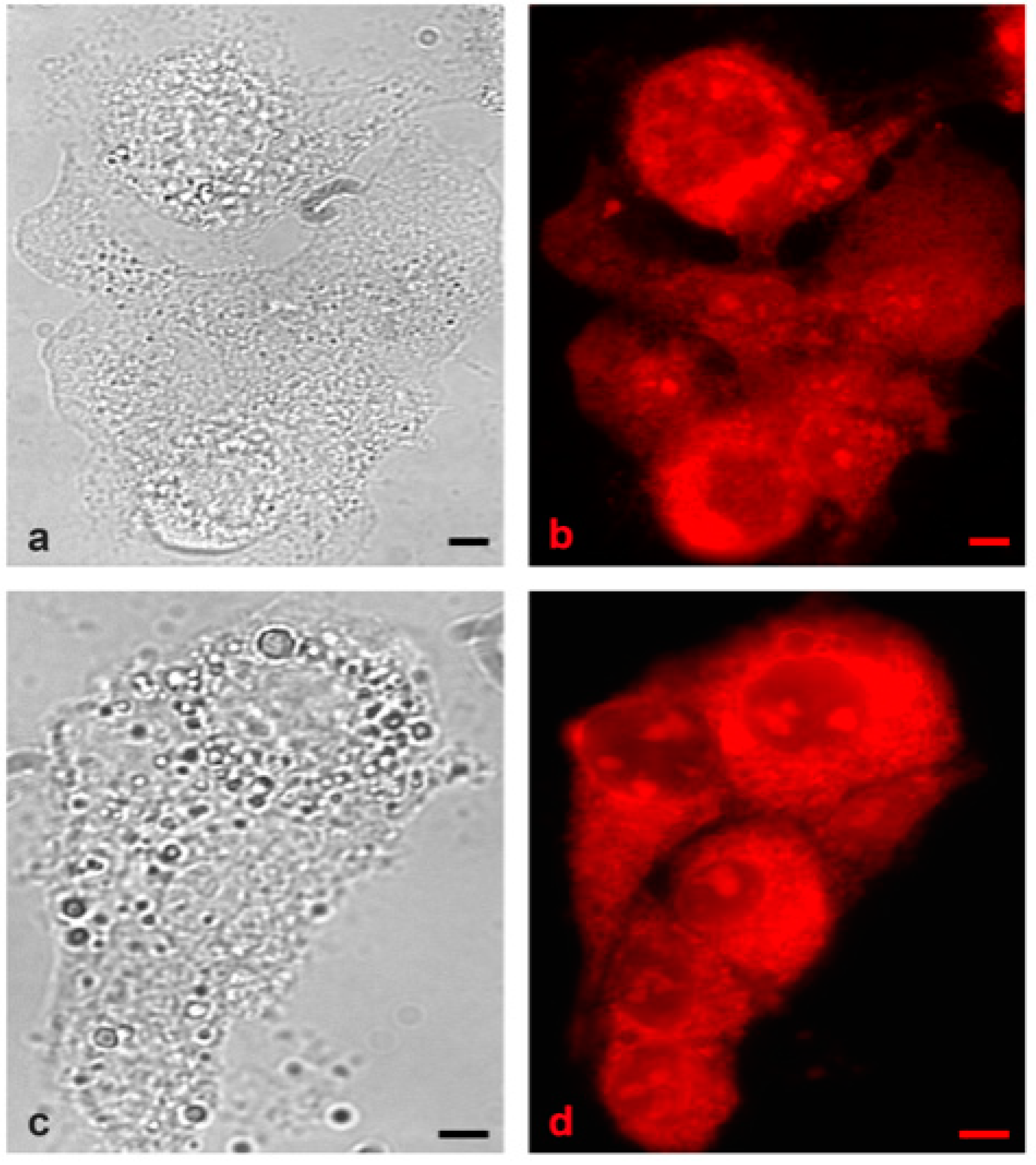
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7.
- Praly, J.-P.; Vidal, S. Inhibition of Glycogen Phosphorylase in the Context of Type 2 Diabetes, with Focus on Recent Inhibitors Bound at the Active Site. Mini-Rev. Med. Chem. 2010, 10, 1102–1126. [Google Scholar] [CrossRef] [PubMed]
- Rousset, M.; Robine-Leon, S.; Dussaulx, E.; Chevalier, G.; Zweibaum, A. Glycogen Storage in Foetal and Malignant Epithelial Cells of the Human Colon. Front. Gastroinest. Res. 1979, 80–85. [Google Scholar] [CrossRef]
- Lee, W.-N.P.; Guo, P.; Lim, S.; Bassilian, S.; Lee, S.T.; Boren, J.; Cascante, M.; Go, V.L.W.; Boros, L.G. Metabolic sensitivity of pancreatic tumour cell apoptosis to glycogen phosphorylase inhibitor treatment. Br. J. Cancer 2004, 91, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Schnier, J.B.; Nishi, K.; Monks, A.; Gorin, F.A.; Bradbury, E.M. Inhibition of glycogen phosphorylase (GP) by CP-91,149 induces growth inhibition correlating with brain GP expression. Biochem. Biophys. Res. Commun. 2003, 309, 126–134. [Google Scholar] [CrossRef]
- Favaro, E.; Harris, A.L. Targeting glycogen metabolism: A novel strategy to inhibit cancer cell growth? Oncotarget 2013, 4, 3–4. [Google Scholar] [CrossRef][Green Version]
- Bak, L.K.; Walls, A.B. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef]
- Swanson, R.A. Brain glycogen—Vestigial no more. Metab. Brain Dis. 2015, 30, 251–253. [Google Scholar] [CrossRef][Green Version]
- Gimisis, T. Synthesis of N-Glucopyranosidic Derivatives as Potential Inhibitors that Bind at the Catalytic Site of Glycogen Phosphorylase. Mini-Rev. Med. Chem. 2010, 10, 1127–1138. [Google Scholar] [CrossRef]
- Mamais, M.; Degli Esposti, A.; Kouloumoundra, V.; Gustavsson, T.; Monti, F.; Venturini, A.; Chrysina, E.D.; Markovitsi, D.; Gimisis, T. A New Potent Inhibitor of Glycogen Phosphorylase Reveals the Basicity of the Catalytic Site. Chem. A Eur. J. 2017, 23, 8800–8805. [Google Scholar] [CrossRef]
- Mamais, M.; Kouloumoundra, V.; Smyrli, E.; Grammatopoulos, P.; Chrysina, E.D.; Gimisis, T. Synthesis of N4-aryl-β-d-glucopyranosylcytosines: A methodology study. Tetrahedron Lett. 2015, 56, 5549–5552. [Google Scholar] [CrossRef][Green Version]
- Haidekker, M.A.; Theodorakis, E.A. Molecular rotors--fluorescent biosensors for viscosity and flow. Org. Biomol. Chem. 2007, 5, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, R.O. Fluorescence probes for polymer free-volume. Pure Appl. Chem. 1986, 58. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Ling, T.; Anglo, M.; Stevens, H.Y.; Frangos, J.A.; Theodorakis, E.A. New fluorescent probes for the measurement of cell membrane viscosity. Chem. Biol. 2001, 8, 123–131. [Google Scholar] [CrossRef]
- Mallipattu, S.; Haidekker, M.; Von Dassow, P.; Latz, M.; Frangos, J. Evidence for shear-induced increase in membrane fluidity in the dinoflagellate Lingulodinium polyedrum. J. Comp. Physiol. A 2002, 188, 409–416. [Google Scholar] [CrossRef]
- Pal, S.; Chakraborty, H.; Bandari, S.; Yahioglu, G.; Suhling, K.; Chattopadhyay, A. Molecular rheology of neuronal membranes explored using a molecular rotor: Implications for receptor function. Chem. Phys. Lipids 2016, 196, 69–75. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Peng, Y.; Cui, X.; Dai, J.; Cui, M. Structure–Property Relationships of Polyethylene Glycol Modified Fluorophore as Near-Infrared Aβ Imaging Probes. Anal. Chem. 2018, 90, 8576–8582. [Google Scholar] [CrossRef]
- Koo, J.Y.; Heo, C.H.; Shin, Y.-H.; Kim, D.; Lim, C.S.; Cho, B.R.; Kim, H.M.; Park, S.B. Readily Accessible and Predictable Naphthalene-Based Two-Photon Fluorophore with Full Visible-Color Coverage. Chem. A Eur. J. 2016, 22, 14166–14170. [Google Scholar] [CrossRef]
- Parrello, D.; Mustin, C.; Brie, D.; Miron, S.; Billard, P. Multicolor Whole-Cell Bacterial Sensing Using a Synchronous Fluorescence Spectroscopy-Based Approach. PLoS ONE 2015, 10, e0122848. [Google Scholar] [CrossRef]
- Koelsch, F. 6-BROMO-2-NAPHTHOL. Org. Synth. 1940, 20, 18. [Google Scholar] [CrossRef]
- Rao, A.S.; Kim, D.; Wang, T.; Kim, K.H.; Hwang, S.; Ahn, K.H. Reaction-based two-photon probes for mercury ions: Fluorescence imaging with dual optical windows. Org. Lett. 2012, 14, 2598–2601. [Google Scholar] [CrossRef]
- Gurrapu, S.; Jonnalagadda, S.K.; Alam, M.A.; Nelson, G.L.; Sneve, M.G.; Drewes, L.R.; Mereddy, V.R. Monocarboxylate transporter 1 inhibitors as potential anticancer agents. ACS Med. Chem. Lett. 2015, 6, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Badía, C.; Souard, F.; Vicent, C. Sugar–Oligoamides: Synthesis of DNA Minor Groove Binders. J. Org. Chem. 2012, 77, 10870–10881. [Google Scholar] [CrossRef] [PubMed]
- Van Ameijde, J.; Albada, H.B.; Liskamp, R.M.J. A convenient preparation of several N-linked glycoamino acid building blocks for efficient solid-phase synthesis of glycopeptides. J. Chem. Soc. Perkin Trans. 1 2002, 2, 1042–1049. [Google Scholar] [CrossRef]
- Pels, K.; Dickson, P.; An, H.; Kodadek, T. DNA-Compatible Solid-Phase Combinatorial Synthesis of β-Cyanoacrylamides and Related Electrophiles. ACS Comb. Sci. 2018, 20, 61–69. [Google Scholar] [CrossRef]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.K.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Oikonomakos, N.G.; Kontou, M.; Zographos, S.E.; Watson, K.A.; Johnson, L.N.; Bichard, C.J.F.; Fleet, G.W.J.; Acharya, K.R. N-acetyl-β-d-glucopyranosylamine: A potent T-state inhibitor of glycogen phosphorylase. A comparison with α-d-glucose. Protein Sci. 1995, 4, 2469–2477. [Google Scholar] [CrossRef]
- Saheki, S.; Takeda, A.; Shimazu, T. Assay of inorganic phosphate in the mild pH range, suitable for measurement of glycogen phosphorylase activity. Anal. Biochem. 1985, 148, 277–281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavreas, K.F.; Mamais, M.; Papazafiri, P.; Gimisis, T. Glucose-Based Molecular Rotors as Fluorescent Inhibitors and Probes of Glycogen Phosphorylase. Chem. Proc. 2021, 3, 45. https://doi.org/10.3390/ecsoc-24-08414
Mavreas KF, Mamais M, Papazafiri P, Gimisis T. Glucose-Based Molecular Rotors as Fluorescent Inhibitors and Probes of Glycogen Phosphorylase. Chemistry Proceedings. 2021; 3(1):45. https://doi.org/10.3390/ecsoc-24-08414
Chicago/Turabian StyleMavreas, Konstantinos F., Michael Mamais, Panagiota Papazafiri, and Thanasis Gimisis. 2021. "Glucose-Based Molecular Rotors as Fluorescent Inhibitors and Probes of Glycogen Phosphorylase" Chemistry Proceedings 3, no. 1: 45. https://doi.org/10.3390/ecsoc-24-08414
APA StyleMavreas, K. F., Mamais, M., Papazafiri, P., & Gimisis, T. (2021). Glucose-Based Molecular Rotors as Fluorescent Inhibitors and Probes of Glycogen Phosphorylase. Chemistry Proceedings, 3(1), 45. https://doi.org/10.3390/ecsoc-24-08414





