1. Introduction
The relationship between properties of aromatic polyamides and the structure of monomers allows for modeling and designing the synthesis of polymers with special properties [
1,
2,
3] for different uses (dialysis membranes and ion exchange systems to thermo-resistant materials and impact protection devices). A singular dependence is observed for aromatic diamines, specifically with their basicity (pKa), which constitutes one of the fundamental criteria for evaluating their reactivities or reaction capacity under catalytic conditions.
Subbotin and Tacoronte [
4] demonstrated the direct dependence between the magnitude of the chemical shifts of the protons of amino groups (NH
2−) and the pKa values. Based on the equation found by them and on the experimental data of the chemical shift values (δ, ppm), the basicity of the primary amino groups, which characterize the reactivity of the monomers at the initial moment of the interphasic polycondensation, can be calculated.
This idea constituted the methodological basis for the study of the influence of various factors on the reactivity (or basicity) of the reacting centers in aromatic diamines with acidic ionogenic substituents (−COOH and −SO
3H). Such substituents must influence the electronic density of the nitrogen atom of the amino group: first, due to its acceptor nature, and second, as a consequence of possible associative processes of intra- and intermolecular hydrogen bonding [
5].
To evaluate this influence, the 1H-NMR spectra of amino-benzoic and aminosulfonic acids and their salts in dimethylacetamide (DMAA) were studied, specifically the values of the chemical shifts (ppm, δ) of the NH2− group because these substituted derivatives constitute simple models of monomers for polyamidation processes in order to obtain thermo-resistant polyamides with excellent properties for membranes. In parallel, for validating the scope of the proposal, we considered evaluating the significance of the net charge on the nitrogen atom, the reaction center in polyamidation processes, when aromatic diamine-type monomers are used.
2. Materials and Methods
The study of the molecules was carried out from molecular designs prepared by the HYPERCHEM-2005 program, and a first optimization of molecular geometry with molecular mechanics was carried out. The quantum mechanical calculations were performed at the semi-empirical level using the AM1 formalism, always setting the PRECISE option that lowers the gradient norm to 0.01 kcal/A (always also using the EF option). To perform the calculation, the Mopac package version 6.0 (MOPAC-6) was used. In this way the net charge of the nitrogen atom in each of the amino groups was calculated. With this result, chemical shift values (δ, ppm) of the hydrogen atom bonded to the nitrogen atom, the amino group (−NH2) can be obtained, from the charge values of the same reported by the MOPAC-6 program, and PM3 method.
To find a model that satisfactorily explains a dependency (structural correlation) between the chemical shift and the charge on the nitrogen atom, we use the ORIGIN program in its version 6.1 (2001–2005) and the STATGRAPHICS program in its version 3.1 (2004–2006).
The synthesis and molecular characterization processes are described in [
6]. The recording of the
1H-NMR spectra was developed in a 200 MHz TESLA equipment, at 25 °C in dimethylacetamide with 2% CaCl
2 or 2% LiCl, using as reference standard TMS (tetramethylsilane, 0.0 ppm, from 0.0 to 12.0 ppm).
The synthetic processes of polysubstituted aromatic diamines, grosso modo, included the use of intermediates from the textile dye industry such as diazo-derivatives and azo-derivatives with various amino (NH2−) or aminoacetoxy (AcONH−) groups or were generated via synthetic methodologies through a sequence of steps that included nitration reactions under eco-sustainable conditions, the insertion of acid ionogenic groups or their precursors, reduction reactions via catalytic hydrogenation and precipitation-isolation for molecular characterization via infrared spectroscopy (FTIR, 4300–500 cm−1, in KBr tablets), thermogravimetry and nuclear magnetic resonance (250 MHz, 0.5 mg analytical sample in CaCl2-dimethylformamide, 12–0.0 ppm, and dimethylsulfoxide-d6).
All the aromatic diamines synthesized are solids with variable coloration depending on the degree of substitution and type of functional groups, and were characterized by their physicochemical properties. Handling procedures, on a laboratory scale, were rigorously considered, given the toxicity and potential carcinogenicity of such organic systems [
6,
7].
3. Results and Discussion
Grosso modo, two basic conceptual positions were used for evaluating the reactivity (basicity, pKa) of the substituted aromatic diamines, used as monomers for polyamidation.
The aromatic diamines under study are depicted in
Figure 1 depending on the procedure used to evaluate the structure–activity correlation: influence of the chemical shift (ppm,
1H-NMR) of the protons (H) bound to the atom of nitrogen in the amino group (NH
2−) on the basicity or reactivity of the aromatic diamine type monomers; and assessment of the net charge correlation on the nitrogen atom vs. chemical shift (δ, ppm) and basicity. All the diamines under study were synthesized according to [
6], under conditions of heterogeneous phase transfer catalysis in aqueous-organic systems of maximum miscibility.
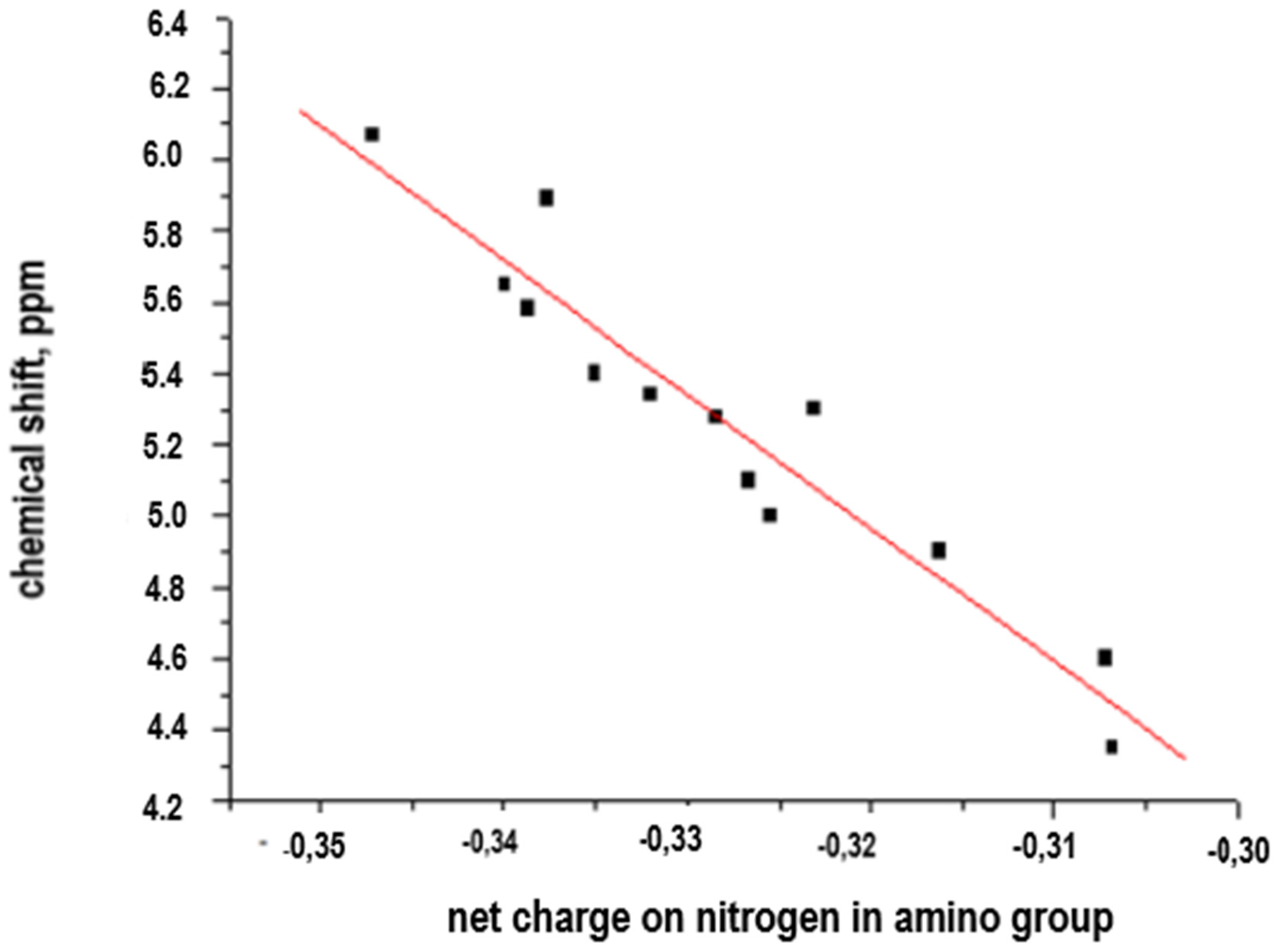
The results of the chemical shift correlation of aromatic amines vs. net charge on the nitrogen atom in NH
2− are shown in
Table 1 and
Figure 2.
A preliminary analysis of the data presented in the
Table 1 and evidence of a potential linear correlation allows us to consider the applicability of a simple system of equations (y = ax + b) and to assess the direct correlation between chemical shift and net charge. The obtained linearity is described in Equation (1).
The Equation (1) that describes the linear correlation of the chemical shift (δ, ppm) vs. net charge on the nitrogen atom in the amino groups is:
Correlation coefficient = −0.957361.
R-Squared = 91.654 %.
Equation (1): Linear correlation of the chemical shift (δ, ppm) vs. net charge on the nitrogen atom in the amino groups.
Considering that the value reported in the ANOVA table is less than 0.01, there is a statistically significant structural relationship between chemical shift and net charge for a 99% confidence level. The R-Squared value of the model explains the 91.654% of the variability of the chemical shift, which allows us to consider this conceptualization valid in order to correlate chemical shift data vs. net charge on reactive center in polyamidation processes from aromatic diamines as monomers for interphasic polycondensation.
The graphic representation of the correlation study for the poly-substituted aromatic diamines represented in
Figure 1 is shown in
Figure 2.
The observed linearity confirms the veracity of the hypothesis about the existence of a direct structure–properties correlation, and its potential applicability in polycondensation processes to determine the reactivity associated with the amino groups (−NH2−) of the aromatic-monomer diamines and potential variability of properties of aromatic polyamides with ionogenic groups (−COOH, −SO3H) and bridging groups (−CH2−, −NH−, −S−, −SO2−, −CONH−) depending on the reactivity of these amino groups (chemical shift, δ, in ppm, net charge on the nitrogen atom for each aromatic diamine). This linearity allows us to consider a satisfactory predictive character for this correlation.
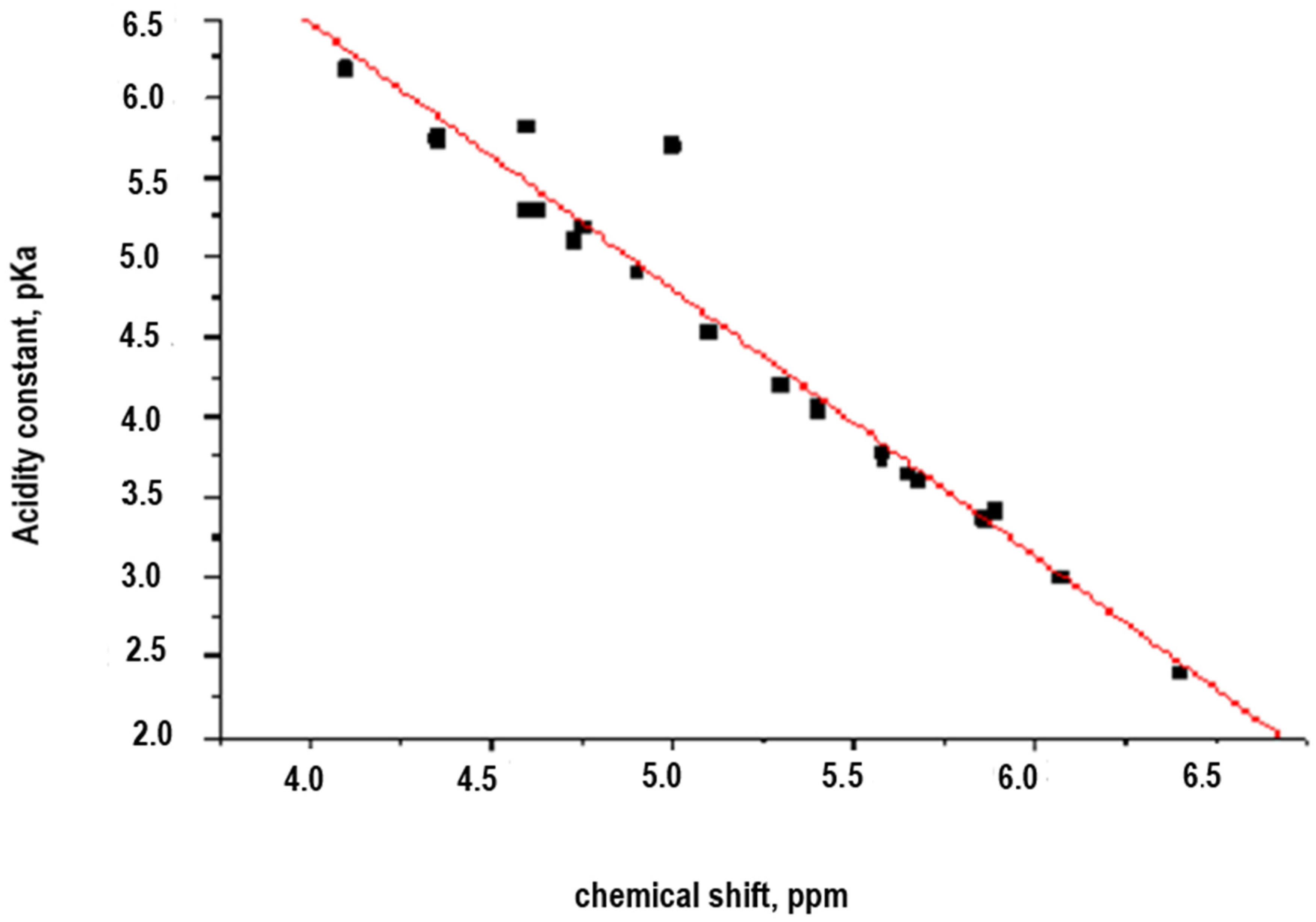
In a second approach, to evaluate the correlation between the structure and the properties of substituted aromatic diamines, the potential dependence between the chemical shift (δ, ppm) and the acidity constant (pKa) of a group of aromatic amines, which defines the reactivity of these, in interphasic polycondensation processes, was evaluated. In this case, used poly-substituted aromatic diamines with ionogenic groups (carboxyl and sulfonic, or in the form of alkaline salts), are represented in
Figure 3. The results are shown in
Table 2 and
Figure 4.
To find a linear correlation between the pKa and the chemical shift (δ, ppm) of the hydrogen atom bonded to the nitrogen atom of the amino group in a series of aromatic amines (
Table 2), the Statgraphics program was used in its version 2.1, because it is easy to manipulate and has a satisfactory degree of freedom and robustness, which allows rapid optimization. The results of the analysis are shown below and the correlation is expressed in Equation (2)
Dependent variable: pKa (basicity or reactivity).
Independent variable: chemical shift, (δ, ppm) of the proton signals (−NH2).
| Parameter | Estimated | Standar Deviation (S) | p Value |
| Intercept | 13.1512 | 0.492256 | 0.0000 |
| Slope | −1.67039 | 0.094198 | 0.0000 |
Correlation coefficient: −0.974017.
R-Squared: 94.8709%.
The Equation (2) for the model is
Equation (2): Linear correlation of the pKa vs. the chemical shift (δ, ppm).
The R-squared value indicates that the model explains the 94.8709% of the variability of the acid constant (pKa). The correlation coefficient of −0.974017 indicates that there is a strong relationship between the variables for a confidence level of 99%.
To evaluate the predictive capacity and potential applicability of this model (procedure 2), the pKa values of some aromatic diamines were determined. The results are shown in
Table 3.
The real value of the pKa of amine 13, determined by the equation, is 4.63, but determined experimentally is 4.53, which is considered within the predicted confidence interval. In the case of amine 12, the real value is 5.30, which only differs by 0.117 from the theoretical value (5.417) and which is also considered within the predicted confidence interval. In the case of the predictions for amine 20 and amine 21, a comparison cannot be made, since the experimental value is not reported in the literature; which supports the possibility of using this procedure and model to predict the potential basicity (pKa), and consequently, reactivity, of these substituted aromatic diamines with ionogenic groups, in poliamidation processes for obtaining ionogenic polyamides with specific properties.