Magnetized Dextrin: Eco-Friendly Effective Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives †
Abstract
:1. Introduction
2. Experimental
2.1. General
2.2. Preparation of Magnetized Dextrin Nanocomposite
2.3. General Procedure for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives
3. Results and Discussion
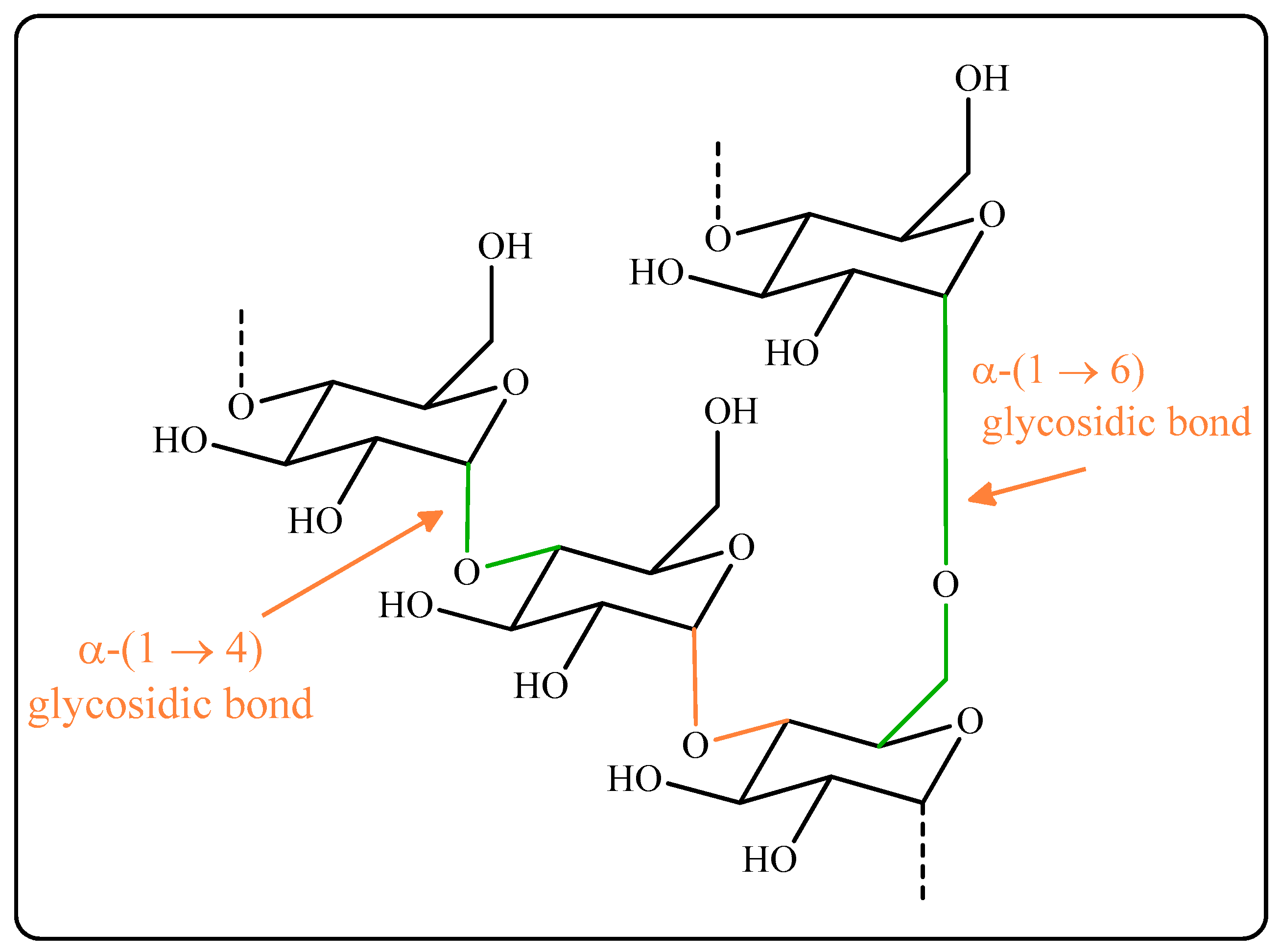
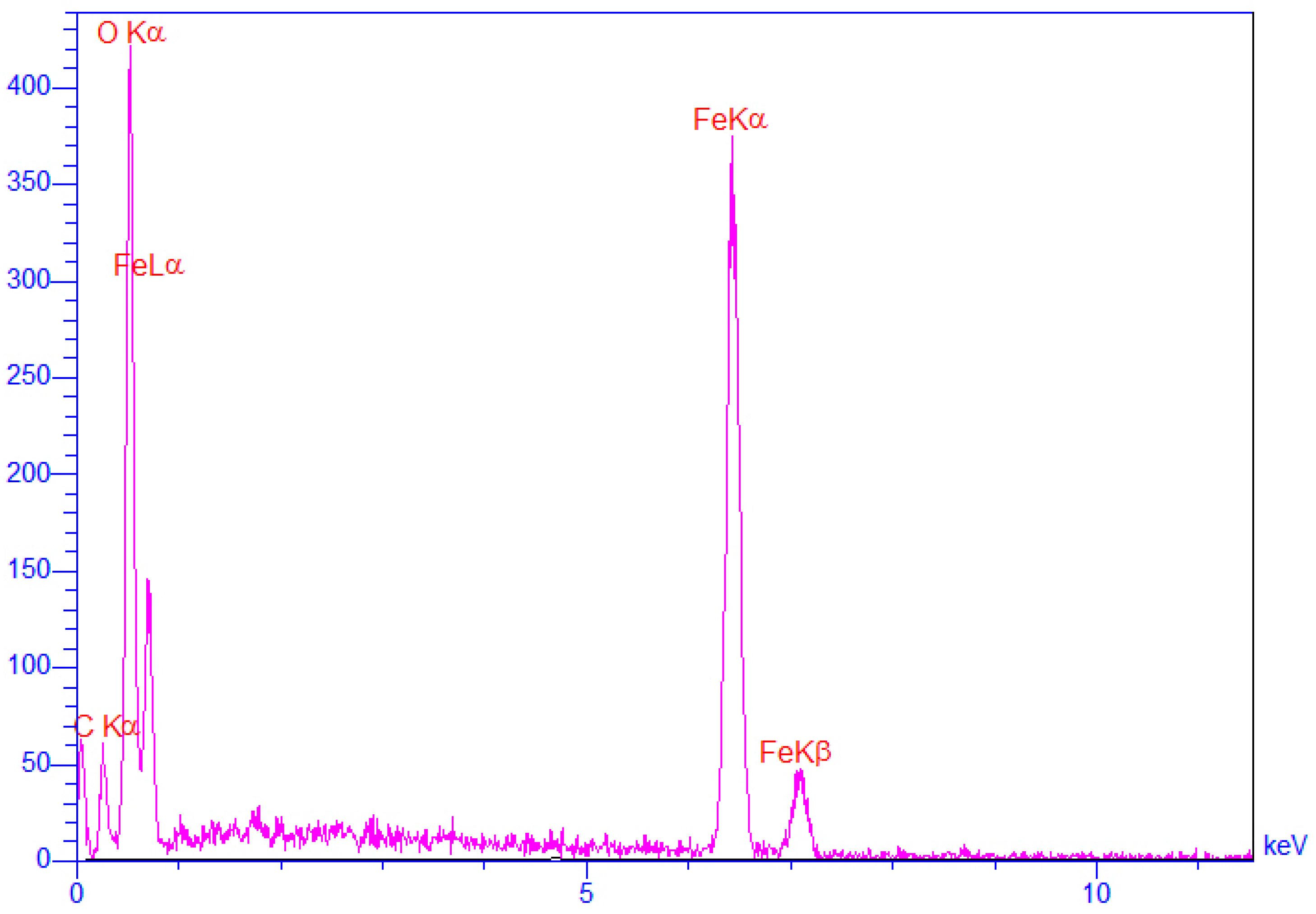
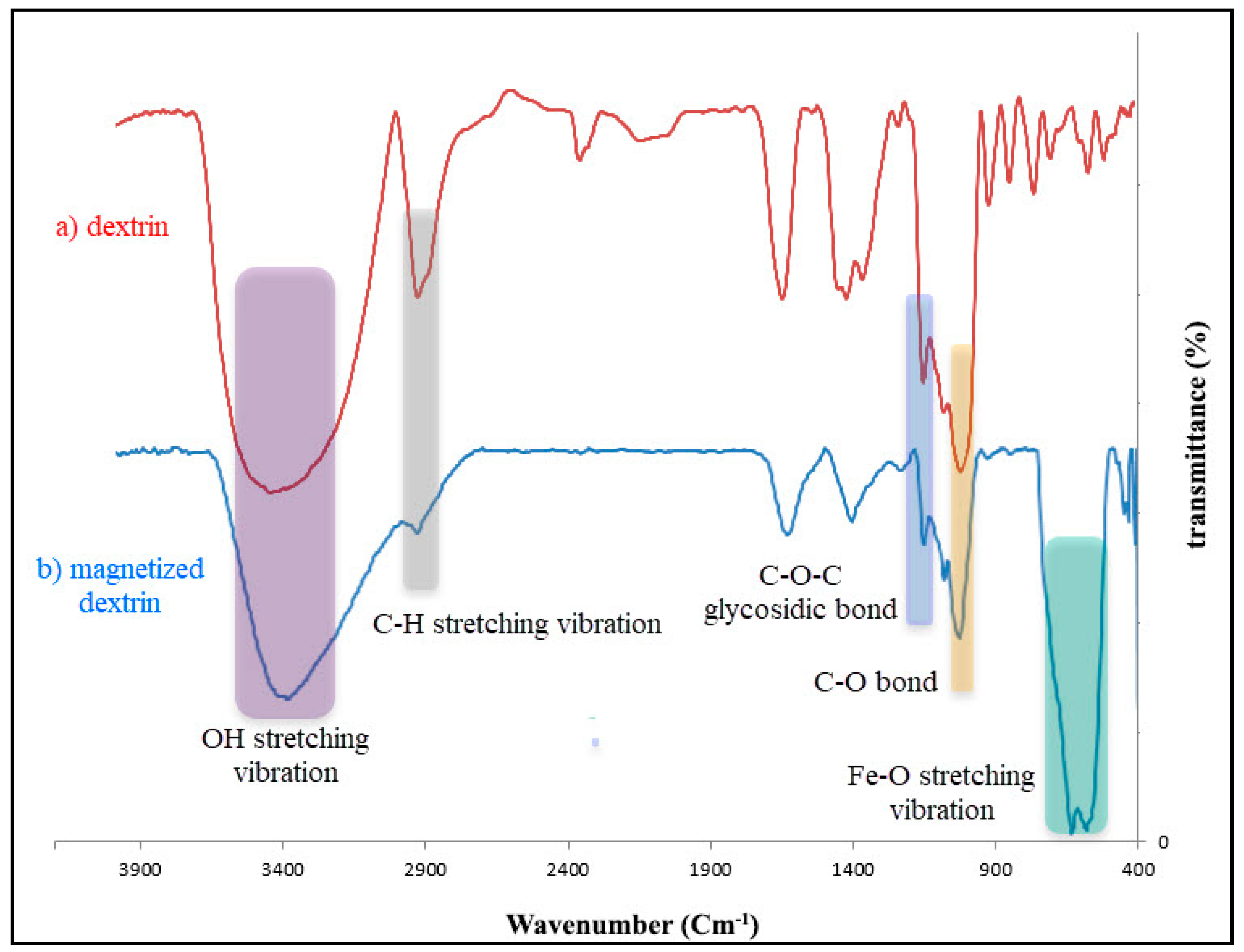
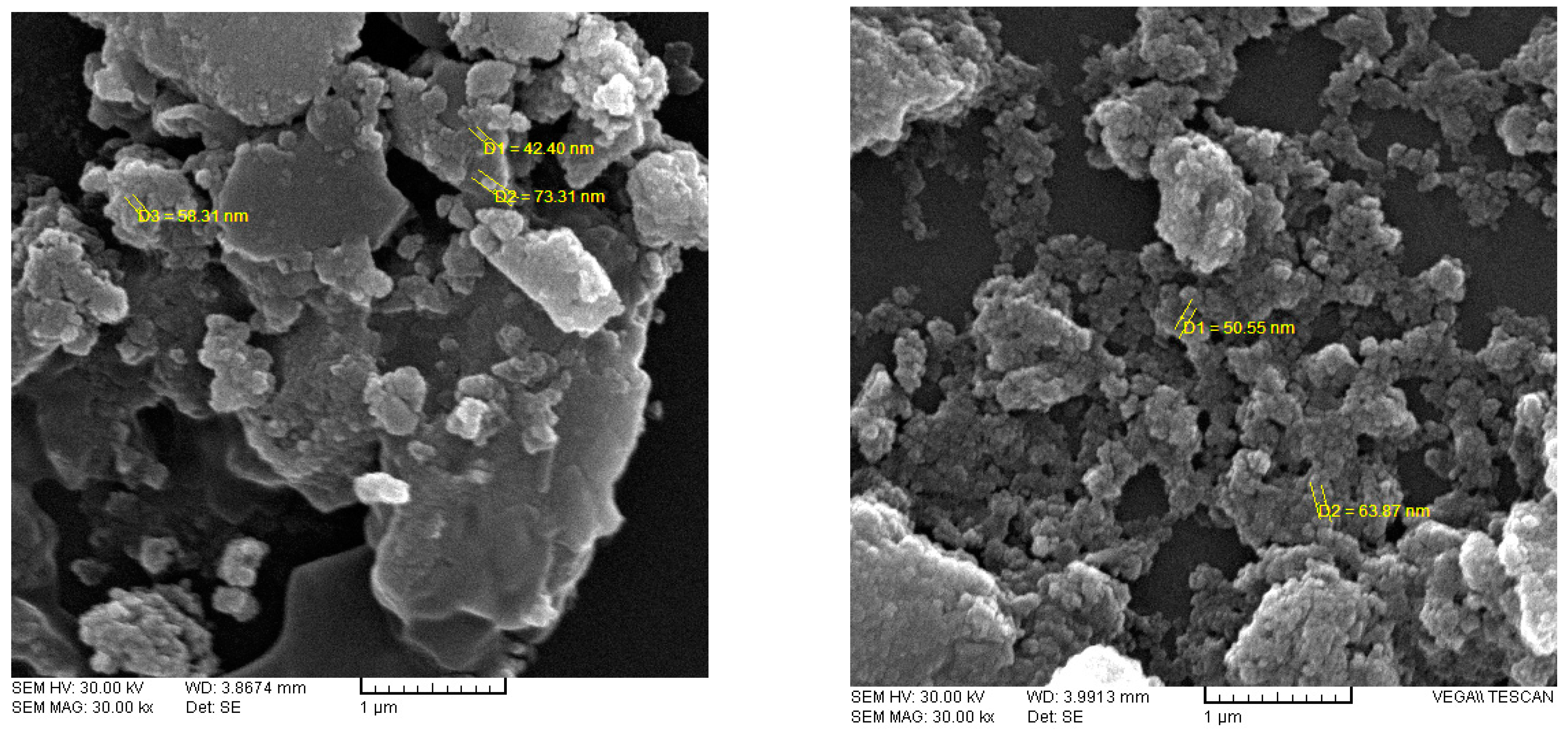
3.1. Characterization Magnetized Dextrin
3.2. Catalytic Application of the Magnetized Dextrin in the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Baran, T.; Baran, N.Y.; Menteş, A. An easily recoverable and highly reproducible agar-supported palladium catalyst for Suzuki-Miyaura coupling reactions and reduction of o-nitroaniline. Int. J. Biol. Macromol. 2018, 115, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nano-biosorbent based on poly m-phenylenediamine grafted dextrin for removal of Pb (II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Hassanzadeh-Afruzi, F.; Varzi, Z.; Esmaeili, M.S. Magnetic dextrin nanobiomaterial: An organic-inorganic hybrid catalyst for the synthesis of biologically active polyhydroquinoline derivatives by asymmetric Hantzsch reaction. Mater. Sci. Eng. C 2020, 109, 110502. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Varzi, Z.; Hassanzadeh-Afruzi, F. Preparation and characterization of an eco-friendly ZnFe2O4@ alginic acid nanocomposite catalyst and its application in the synthesis of 2-amino-3-cyano-4H-pyran derivatives. Polyhedron 2019, 171, 193–202. [Google Scholar] [CrossRef]
- Hajizadeh, Z.; Maleki, A. Poly (ethylene imine)-modified magnetic halloysite nanotubes: A novel, efficient and recyclable catalyst for the synthesis of dihydropyrano [2,3-c] pyrazole derivatives. Mol. Catal. 2018, 460, 87–93. [Google Scholar] [CrossRef]
- Maleki, A.; Firouzi-Haji, R.; Hajizadeh, Z. Magnetic guanidinylated chitosan nanobiocomposite: A green catalyst for the synthesis of 1, 4-dihydropyridines. Int. J. Biol. Macromol. 2018, 116, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Jafari, A.A.; Yousefi, S. Green cellulose-based nanocomposite catalyst: Design and facile performance in aqueous synthesis of pyranopyrimidines and pyrazolopyranopyrimidines. Carbohydr. Polym. 2017, 175, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Hassanzadeh-Afruzi, F.; Maleki, A. Synthesis and characterization of a novel and green rod-like magnetic ZnS/CuFe2O4/agar organometallic hybrid catalyst for the synthesis of biologically-active 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives. Appl. Organomet. Chem. 2020, 34, e5949. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M. Biodegradable polyaniline/dextrin conductive nanocomposites: Synthesis, characterization, and study of antioxidant activity and sorption of heavy metal ions. Iran. Polym. J. 2014, 23, 257–266. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Taheri-Ledari, R.; Khosropour, N.; Dalvand, S.; Maleki, A.; Mousavi-Khoshdel, S.M.; Sohrabi, H. Fe3O4/GO@ melamine-ZnO nanocomposite: A promising versatile tool for organic catalysis and electrical capacitance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124335. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri-Khamakani, Z.; Hassanzadeh-Afruzi, F.; Maleki, A. Magnetized Dextrin: Eco-Friendly Effective Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives. Chem. Proc. 2021, 3, 101. https://doi.org/10.3390/ecsoc-24-08285
Amiri-Khamakani Z, Hassanzadeh-Afruzi F, Maleki A. Magnetized Dextrin: Eco-Friendly Effective Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives. Chemistry Proceedings. 2021; 3(1):101. https://doi.org/10.3390/ecsoc-24-08285
Chicago/Turabian StyleAmiri-Khamakani, Zeinab, Fereshte Hassanzadeh-Afruzi, and Ali Maleki. 2021. "Magnetized Dextrin: Eco-Friendly Effective Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives" Chemistry Proceedings 3, no. 1: 101. https://doi.org/10.3390/ecsoc-24-08285
APA StyleAmiri-Khamakani, Z., Hassanzadeh-Afruzi, F., & Maleki, A. (2021). Magnetized Dextrin: Eco-Friendly Effective Nanocatalyst for the Synthesis of Dihydropyrano[2,3-c]pyrazole Derivatives. Chemistry Proceedings, 3(1), 101. https://doi.org/10.3390/ecsoc-24-08285







