Combined DFT and Operando Spectroscopic Study of the Water-Gas Shift Reaction over Ceria-Based Catalysts: The Role of the Noble Metal and Ceria Faceting †
Abstract
:1. Introduction
2. Methods
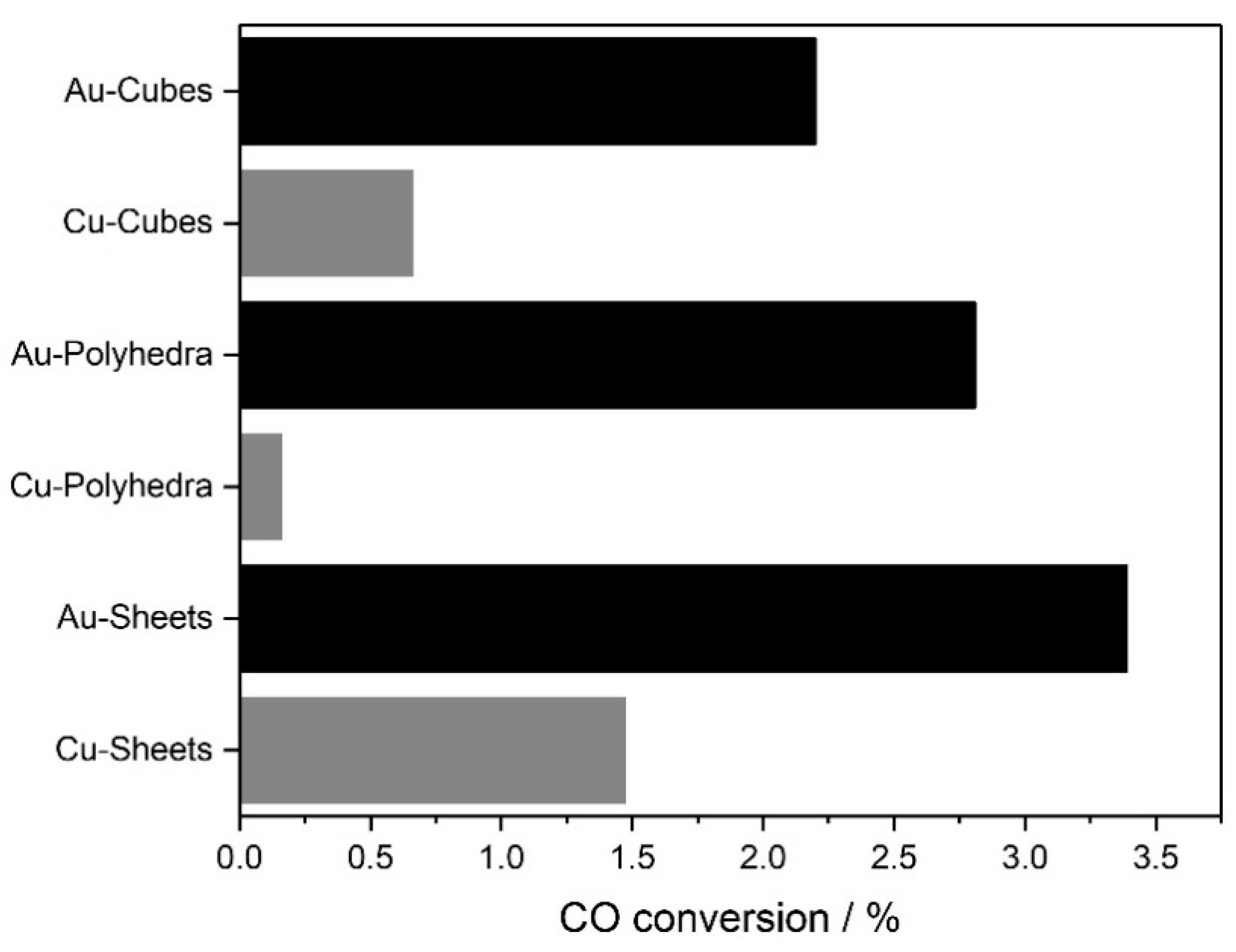
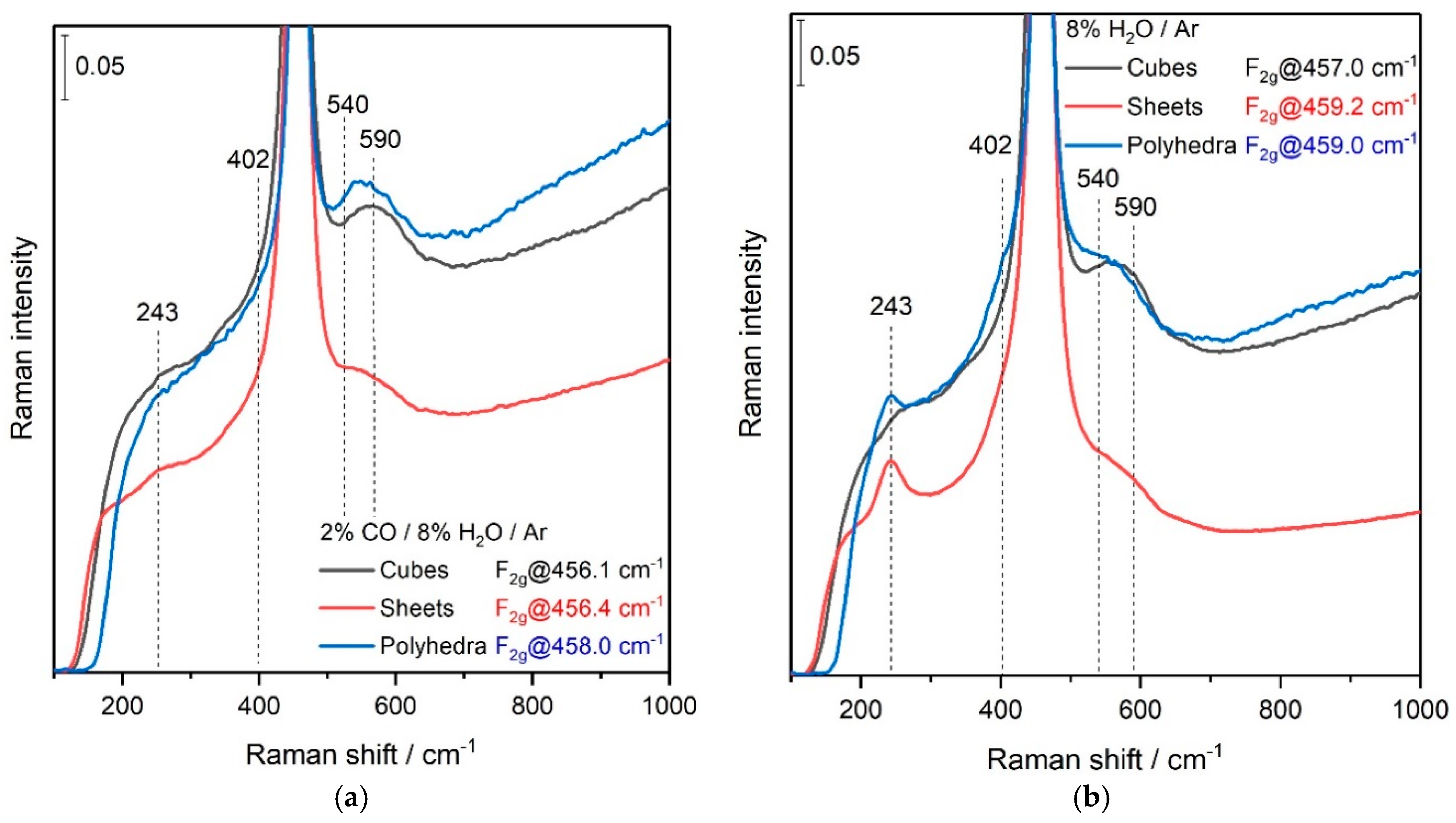
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Trimm, D.L. Minimisation of carbon monoxide in a hydrogen stream for fuel cell application. Appl. Catal. A Gen. 2005, 296, 1–11. [Google Scholar] [CrossRef]
- Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-dependent properties of Cu/CeO2 catalysts for the water-gas shift reaction. Catalysts 2017, 7. [Google Scholar] [CrossRef]
- Schilling, C.; Hess, C. Elucidating the Role of Support Oxygen in the Water–Gas Shift Reaction over Ceria-Supported Gold Catalysts Using Operando Spectroscopy. ACS Catal. 2019, 9, 1159–1171. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Ziemba, M.; Ganduglia-Pirovano, V.; Hess, C. Insight into the mechanism of the water-gas shift reaction over Au/CeO2 catalysts using combined operando spectroscopies. Faraday Discuss. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Burch, R.; Hardacre, C.; Hu, P. New insight into mechanisms in water-gas-shift reaction on Au/CeO2(111): A density functional theory and kinetic study. Faraday Discuss. 2011, 152, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, M.; Hess, C. Influence of gold on the reactivity behaviour of ceria nanorods in CO oxidation: Combining operando spectroscopies and DFT calculations. Catal. Sci. Technol. 2020, 10, 3720–3730. [Google Scholar] [CrossRef]
- Nottbohm, C.T.; Hess, C. Investigation of ceria by combined Raman, UV–vis and X-ray photoelectron spectroscopy. Catal. Commun. 2012, 22, 39–42. [Google Scholar] [CrossRef]
- Schilling, C.; Hess, C. Real-Time Observation of the Defect Dynamics in Working Au/CeO2 Catalysts by Combined Operando Raman/UV–Vis Spectroscopy. J. Phys. Chem. C 2018, 122, 2909–2917. [Google Scholar] [CrossRef]
- Kim, H.Y.; Henkelman, G. CO Oxidation at the Interface of Au Nanoclusters and the Stepped-CeO2(111) Surface by the Mars–van Krevelen Mechanism. J. Phys. Chem. Lett. 2013, 4, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the Mechanism of Low-Temperature Water Gas Shift Reaction on Copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemba, M.; Stark, D.; Hess, C. Combined DFT and Operando Spectroscopic Study of the Water-Gas Shift Reaction over Ceria-Based Catalysts: The Role of the Noble Metal and Ceria Faceting. Chem. Proc. 2020, 2, 23. https://doi.org/10.3390/ECCS2020-07531
Ziemba M, Stark D, Hess C. Combined DFT and Operando Spectroscopic Study of the Water-Gas Shift Reaction over Ceria-Based Catalysts: The Role of the Noble Metal and Ceria Faceting. Chemistry Proceedings. 2020; 2(1):23. https://doi.org/10.3390/ECCS2020-07531
Chicago/Turabian StyleZiemba, Marc, Danny Stark, and Christian Hess. 2020. "Combined DFT and Operando Spectroscopic Study of the Water-Gas Shift Reaction over Ceria-Based Catalysts: The Role of the Noble Metal and Ceria Faceting" Chemistry Proceedings 2, no. 1: 23. https://doi.org/10.3390/ECCS2020-07531
APA StyleZiemba, M., Stark, D., & Hess, C. (2020). Combined DFT and Operando Spectroscopic Study of the Water-Gas Shift Reaction over Ceria-Based Catalysts: The Role of the Noble Metal and Ceria Faceting. Chemistry Proceedings, 2(1), 23. https://doi.org/10.3390/ECCS2020-07531




