Multicomponent Synthesis of the New Compound 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(4-pyridin-2-yl)phenyl)-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one †
Abstract
1. Introduction
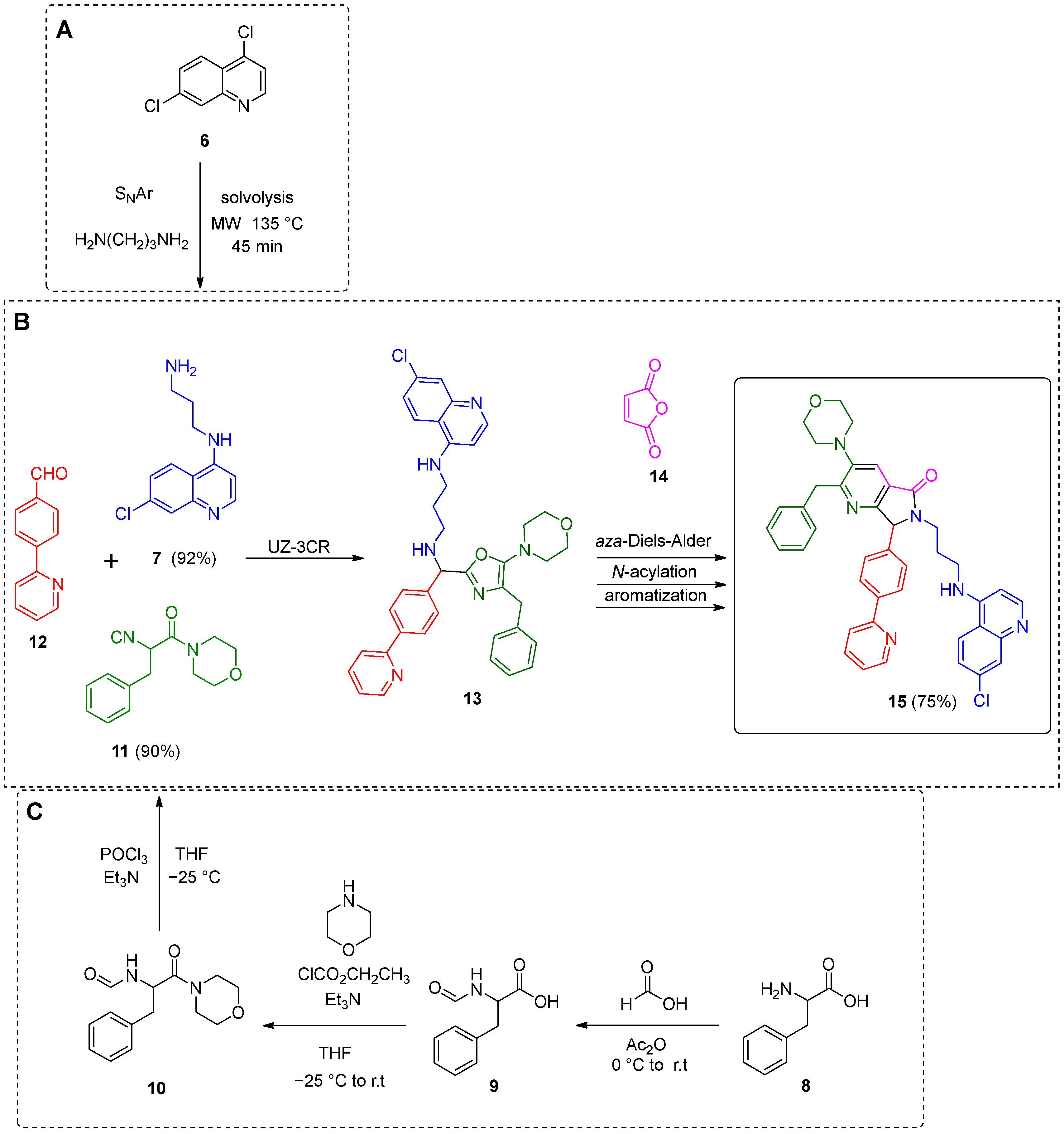
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation and Chemicals
3.2. General Procedure
3.3. Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibarra, I.A.; Islas-Jacome, A.; Gonzalez-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Janvier, P.; Zhao, G.; Bienayme, H.; Zhu, J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Devasthale, P.; Wang, Y.; Wang, W.; Fevig, J.; Feng, J.; Wang, A.; Harrity, T.; Egan, D.; Morgan, N.; Cap, M.; et al. Optimization of Activity, Selectivity, and Liability Profiles in 5-Oxopyrrolopyridine DPP4 Inhibitors Leading to Clinical Candidate (Sa)-2-(3-(Aminomethyl)-4-(2,4-dichlorophenyl)-2-methyl-5-oxo-5H-pyrrolo [3,4-b] pyridin-6(7H)-yl)-N,N-dimethylacetamide (BMS-767778). J. Med. Chem. 2013, 56, 7343–7357. [Google Scholar] [PubMed]
- Segura-Olvera, D.; García-González, A.N.; Morales-Salazar, I.; Islas-Jácome, A.; Rojas-Aguirre, Y.; Ibarra, I.A.; Díaz-Cervantes, E.; Alcaraz-Estrada, S.L.; González-Zamora, E. Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Multicomponent Reactions and In Vitro–In Silico Studies Against SiHa, HeLa, and CaSki Human Cervical Carcinoma Cell Lines. Molecules 2019, 24, 2648. [Google Scholar] [CrossRef] [PubMed]
- Morales-Salazar, I.; Garduño-Albino, C.E.; Montes-Enríquez, F.P.; Nava-Tapia, D.A.; Navarro-Tito, N.; Herrera-Zúñiga, L.D.; González-Zamora, E.; Islas-Jácome, A. Synthesis of Pyrrolo[3,4-b]pyridin-5-ones via Ugi–Zhu Reaction and In Vitro–In Silico Studies against Breast Carcinoma. Pharmaceuticals 2023, 16, 1562. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Carapia, R.E.; Hernández-López, R.; Alcaraz-Estrada, S.L.; Sarmiento-Silva, R.E.; García-Hernández, M.E.; Estrada-Toledo, N.V.; Padilla-Bernal, G.; Herrera-Zúñiga, L.D.; Garza, J.; Vargas, R.; et al. Multi-Component Synthesis of New Fluorinated-Pyrrolo[3,4-b]pyridin-5-ones Containing the 4-Amino-7-chloroquinoline Moiety and In Vitro–In Silico Studies Against Human SARS-CoV-2. Int. J. Mol. Sci. 2025, 26, 7651. [Google Scholar] [CrossRef] [PubMed]
- Flores-Reyes, J.C.; Amador-Sanchez, Y.A.; Valderrama-Celestino, A.; Barrios-Campos, B.D.; Peralta, R.A.; Huxley, M.T.; Ibarra, I.A.; Islas-Jácome, A.; Solis-Ibarra, D.; Gonzalez-Zamora, E. Dual-state emission of pyrazolyl-pyrrolo[3,4-b]pyridin-5-ones via excited-state intramolecular proton transfer (ESIPT): Multicomponent synthesis and optical characterization. Org. Chem. Front. 2025, 12, 2607–2616. [Google Scholar] [CrossRef]
- Craig, C.A.; Garces, F.O.; Watts, R.J.; Palmans, R.; Frank, A.J. Luminescence properties of two new Pt(II)-2-phenylpyridine complexes; the influence of metal-carbon bonds. Coord. Chem. Rev. 1990, 97, 193–208. [Google Scholar] [CrossRef]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienayme, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their Use in the Multicomponent Synthesis of 5-Aminooxazole, Pyrrolo[3,4-b]pyridin-5-one and 4,5,6,7-Tetrahydrofuro [2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
- Saito, T.; Kawawura, M.; Nishimura, J. Ytterbium triflate-catalyzed asymmetric hetero Diels-Alder cycloaddition of a 1-thiabuta-1,3-diene with a chiral N-acryloyloxazolidinone dienophile. Diastereoface control by solvents or achiral additives. Tetrahedron. Lett. 1997, 38, 3231–3234. [Google Scholar] [CrossRef]


| Entry | % mol Yb(OTf)3 | Yield (%) a |
|---|---|---|
| 1 | 5% | 51 |
| 2 | 7% | 64 |
| 3 | 10% | 75 |
| 4 | 12% | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Carapia, R.E.; Alonso-Pérez, R.; Islas-Jácome, A.; Gonzalez-Zamora, E. Multicomponent Synthesis of the New Compound 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(4-pyridin-2-yl)phenyl)-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one. Chem. Proc. 2025, 18, 93. https://doi.org/10.3390/ecsoc-29-26676
Blanco-Carapia RE, Alonso-Pérez R, Islas-Jácome A, Gonzalez-Zamora E. Multicomponent Synthesis of the New Compound 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(4-pyridin-2-yl)phenyl)-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one. Chemistry Proceedings. 2025; 18(1):93. https://doi.org/10.3390/ecsoc-29-26676
Chicago/Turabian StyleBlanco-Carapia, Roberto E., Rodolfo Alonso-Pérez, Alejandro Islas-Jácome, and Eduardo Gonzalez-Zamora. 2025. "Multicomponent Synthesis of the New Compound 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(4-pyridin-2-yl)phenyl)-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one" Chemistry Proceedings 18, no. 1: 93. https://doi.org/10.3390/ecsoc-29-26676
APA StyleBlanco-Carapia, R. E., Alonso-Pérez, R., Islas-Jácome, A., & Gonzalez-Zamora, E. (2025). Multicomponent Synthesis of the New Compound 2-Benzyl-6-(3-((7-chloroquinolin-4-yl)amino)propyl)-3-morpholino-7-(4-pyridin-2-yl)phenyl)-6,7-dihidro-5H-pyrrolo[3,4-b]pyridin-5-one. Chemistry Proceedings, 18(1), 93. https://doi.org/10.3390/ecsoc-29-26676






