Synthesis of Bis-1,3,4-Oxadiazoles Utilizing Monomers Derived from the Degradation of PET (Polyethylene Terephthalate) in an Eco-Friendly Manner †
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information, Software Instrumentation and Chemicals
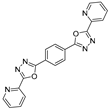
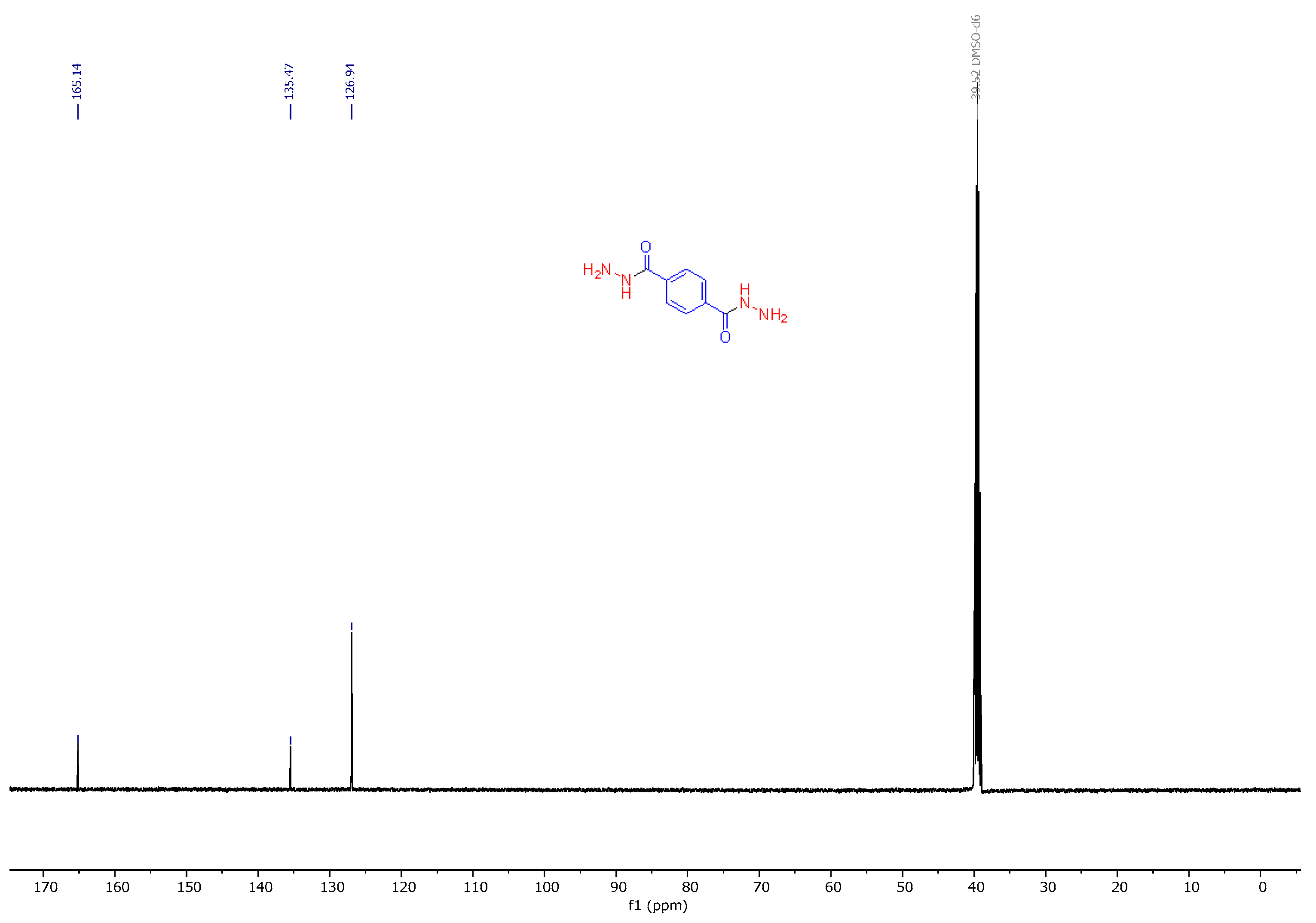
3.2. Synthesis and Characterization of 2,5-Diaryl-bis-1,3,4-oxadiazoles
3.3. Spectral Data
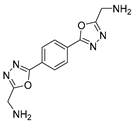
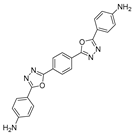

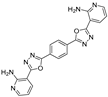
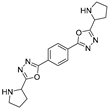
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, N.; Kurian, T. Recent Developments in the Chemical Recycling of Postconsumer Poly(Ethylene Terephthalate) Waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef] [PubMed]
- More, A.P.; Kute, R.A.; Mhaske, S.T. Chemical Conversion of PET Waste Using Ethanolamine to Bis(2-Hydroxyethyl) Terephthalamide (BHETA) through Aminolysis and a Novel Plasticizer for PVC. Iran. Polym. J. 2014, 23, 59–67. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on Methanolytic Depolymerization of PET with Supercritical Methanol for Chemical Recycling. Polym. Degrad. Stab. 2002, 75, 185–191. [Google Scholar] [CrossRef]
- Gupta, P.; Bhandari, S. 6-Chemical Depolymerization of PET Bottles via Ammonolysis and Aminolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 109–134. ISBN 978-0-12-811361-5. [Google Scholar]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical Recycling of PET Wastes with Different Catalysts. Int. J. Polym. Sci. 2015, 2015, 124524. [Google Scholar] [CrossRef]
- Kumar, D.; Dalai, S.; Chandra Sharma, G.; Jangir, M. Synthesis of a Novel 1,3,4-Oxadiazole Derivative with Piperazine and Phenoxypropanol Functionalities. ACS Omega 2025, 10, 19033–19044. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Kamboj, S.; Kajal, A.; Saini, V.; Prasad, D.N. 1,3,4-Oxadiazole Derivatives: Synthesis, Characterization, Antimicrobial Potential, and Computational Studies. BioMed Res. Int. 2014, 2014, 172791. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, K.; Qarah, A.F.; Alharbi, H.; Alisaac, A.; Abualnaja, M.M.; Attar, R.M.S.; Alsoliemy, A.; El-Metwaly, N.M. Synthesis of Designed New 1,3,4-Oxadiazole Functionalized Pyrano [2,3-f] Chromene Derivatives and Their Antimicrobial Activities. Heliyon 2024, 10, e38294. [Google Scholar] [CrossRef] [PubMed]
- Nargund, L.V.G.; Reddy, G.R.N.; Hariprasad, V. Anti-Inflammatory Activity of Substituted 1,3,4-Oxadiazoles. J. Pharm. Sci. 1994, 83, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Almasirad, A.; Mousavi, Z.; Tajik, M.; Assarzadeh, M.J.; Shafiee, A. Synthesis, Analgesic and Anti-Inflammatory Activities of New Methyl-Imidazolyl-1,3,4-Oxadiazoles and 1,2,4-Triazoles. DARU J. Pharm. Sci. 2014, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Hameed, S.; Al-Masoudi, N.; Loddo, R.; Colla, P. In Vitro Antitumor and Antiviral Activities of New Benzothiazole and 1,3,4-Oxadiazole-2-Thione Derivatives. Acta Pharm. 2008, 58, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Capoci, I.R.G.; Sakita, K.M.; Faria, D.R.; Rodrigues-Vendramini, F.A.V.; Arita, G.S.; de Oliveira, A.G.; Felipe, M.S.; Maigret, B.; Bonfim-Mendonça, P.d.S.; Kioshima, E.S.; et al. Two New 1,3,4-Oxadiazoles with Effective Antifungal Activity Against Candida Albicans. Front. Microbiol. 2019, 10, 2130. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.A.; Yar, M.S.; Abdel-Hamid, S.G.; Al Qasoumi, S.I.; Samad, A. Molecular Properties Prediction, Synthesis and Antimicrobial Activity of Some Newer Oxadiazole Derivatives. Eur. J. Med. Chem. 2010, 45, 5862–5869. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Tarannum, N.; Chourasia, M.; Soni, R.K. Chemical Degradation of Poly(Ethylene Terephthalate) for Potential Antimicrobial Activity Evaluation and Molecular Docking Study. J. Polym. Environ. 2018, 26, 819–829. [Google Scholar] [CrossRef]


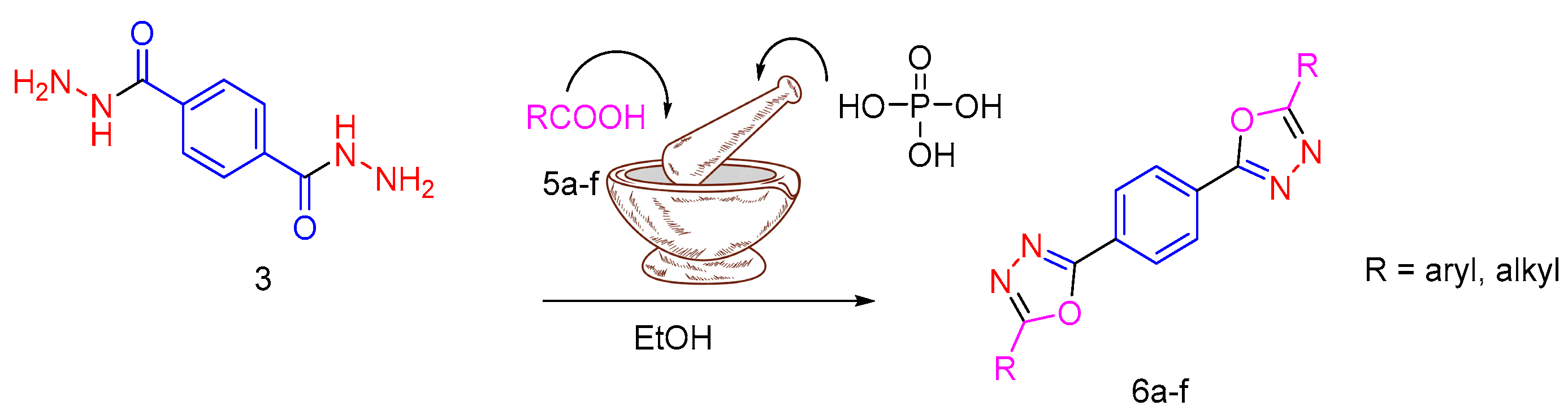

| RCOOH | Oxadiazole | %Yield |
|---|---|---|
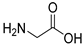 |  | 88 |
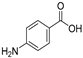 |  | 72 |
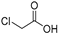 |  | 50 |
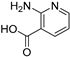 | 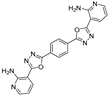 | 85 |
 |  | 93 |
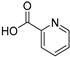 | 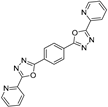 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara, J.G.; Unnamatla, M.V.B.; Yañez, E.C.; Becerril, D.C.; Eleno, M.A.G. Synthesis of Bis-1,3,4-Oxadiazoles Utilizing Monomers Derived from the Degradation of PET (Polyethylene Terephthalate) in an Eco-Friendly Manner. Chem. Proc. 2025, 18, 5. https://doi.org/10.3390/ecsoc-29-26670
Guevara JG, Unnamatla MVB, Yañez EC, Becerril DC, Eleno MAG. Synthesis of Bis-1,3,4-Oxadiazoles Utilizing Monomers Derived from the Degradation of PET (Polyethylene Terephthalate) in an Eco-Friendly Manner. Chemistry Proceedings. 2025; 18(1):5. https://doi.org/10.3390/ecsoc-29-26670
Chicago/Turabian StyleGuevara, Jareth García, Murali Venkata Basavanag Unnamatla, Erick Cuevas Yañez, David Corona Becerril, and Marco Antonio García Eleno. 2025. "Synthesis of Bis-1,3,4-Oxadiazoles Utilizing Monomers Derived from the Degradation of PET (Polyethylene Terephthalate) in an Eco-Friendly Manner" Chemistry Proceedings 18, no. 1: 5. https://doi.org/10.3390/ecsoc-29-26670
APA StyleGuevara, J. G., Unnamatla, M. V. B., Yañez, E. C., Becerril, D. C., & Eleno, M. A. G. (2025). Synthesis of Bis-1,3,4-Oxadiazoles Utilizing Monomers Derived from the Degradation of PET (Polyethylene Terephthalate) in an Eco-Friendly Manner. Chemistry Proceedings, 18(1), 5. https://doi.org/10.3390/ecsoc-29-26670







