Sucralose Disrupts LuxR-Type Quorum Sensing: Implications for Anti-Cariogenic Activity †
Abstract
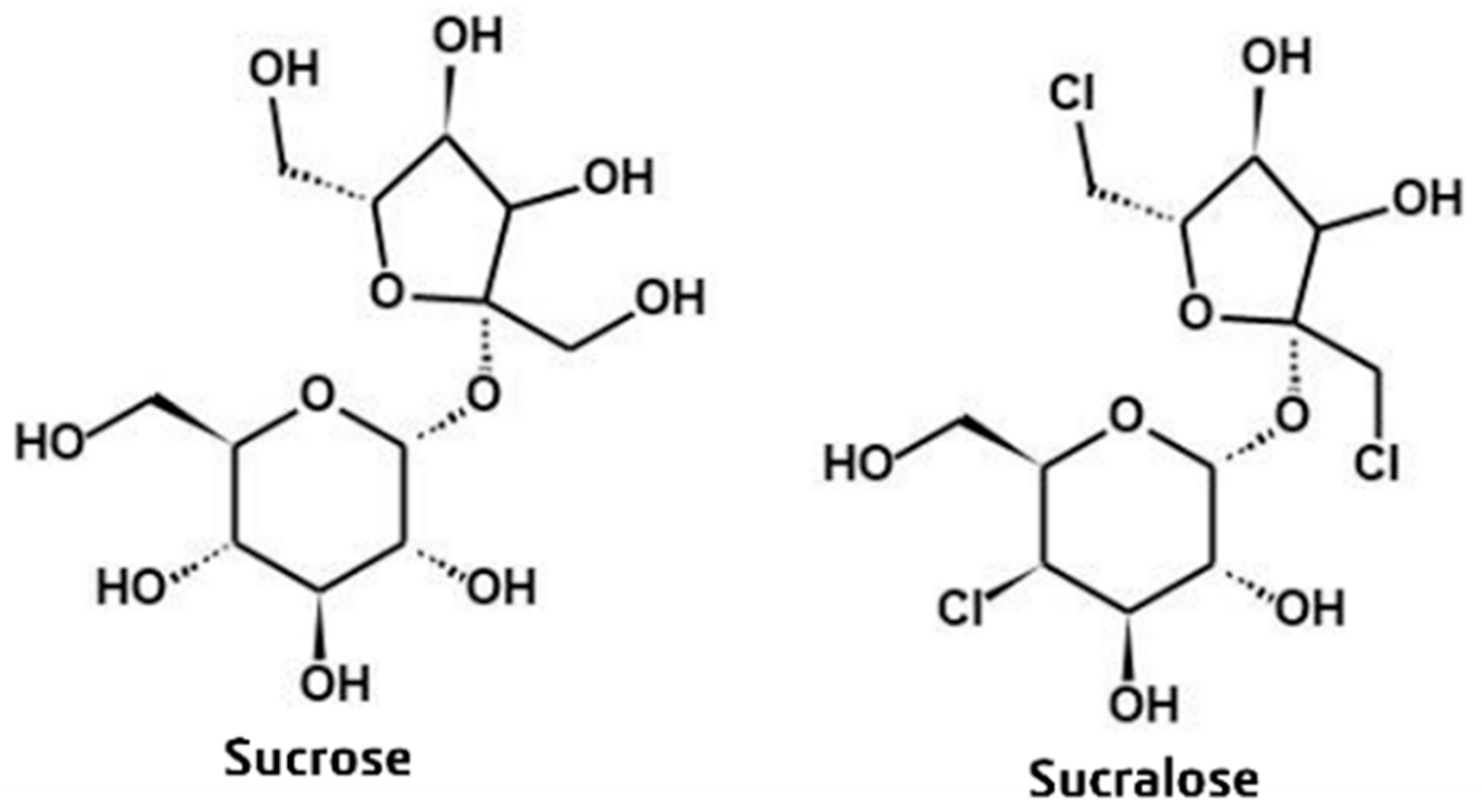
1. Introduction
2. Methods
2.1. Bacterial Cultivation
2.2. Protein Expression
2.3. Protein Purification and Determination
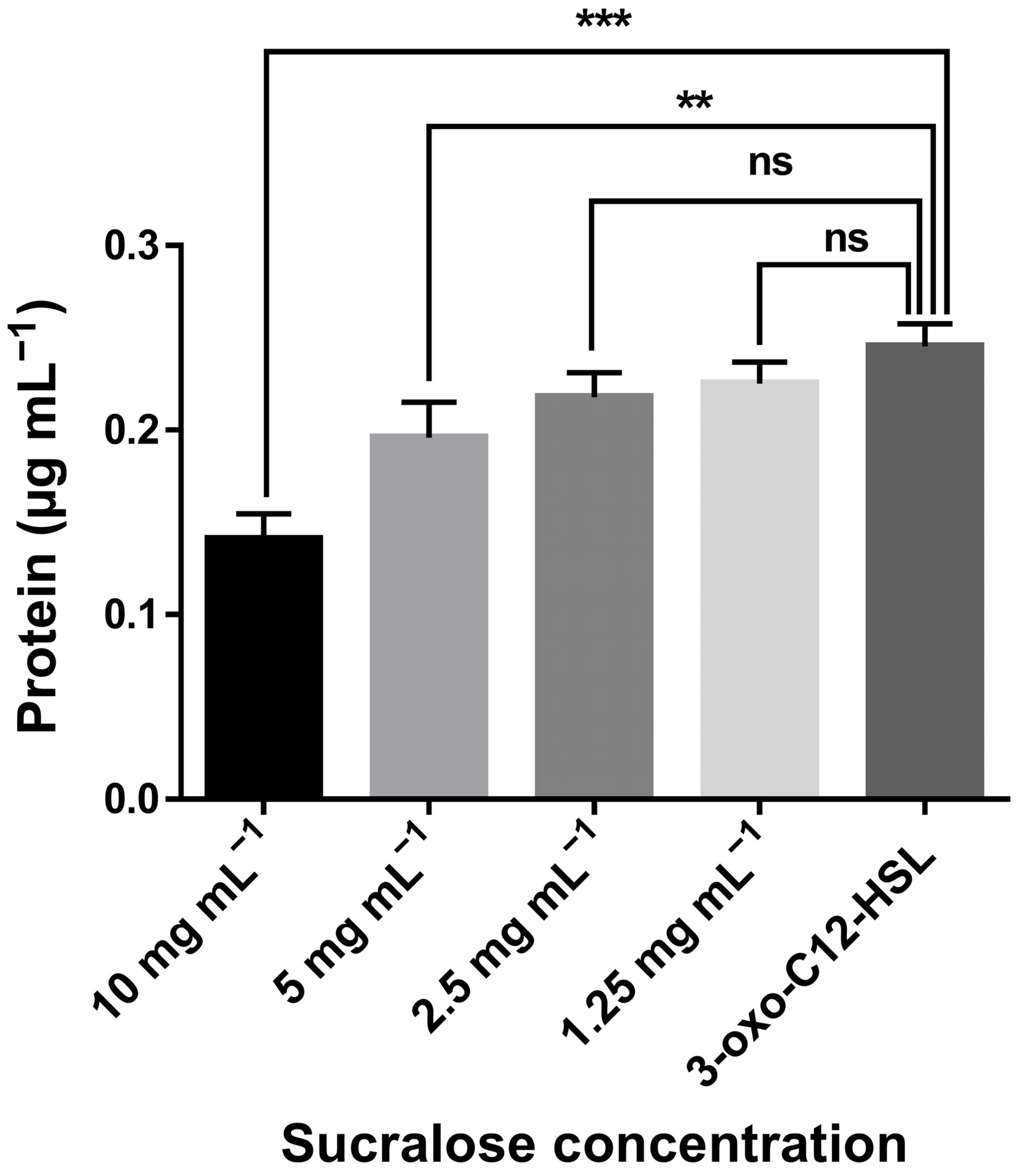
3. Result
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QS | Quorum sensing |
| AHL | N-acyl homoserine lactone |
| NTA | Nitriloacetic acid |
| DMSO | Dimethyl sulfoxide |
| FDA | The Food and Drug Administration of the United States |
| DDW | Double-distilled water |
| PMSF | Phenylmethylsulfonyl fluoride |
References
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Rother, K.I. Sucralose, A Synthetic Organochlorine Sweetener: Overview Of Biological Issues. J. Toxicol. Environ. Heal Part B 2013, 16, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Glória, M.B.A. SWEETENERS|Others. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5695–5702. ISBN 9780122270550. [Google Scholar]
- Aguayo-Guerrero, J.A.; Méndez-García, L.A.; Solleiro-Villavicencio, H.; Viurcos-Sanabria, R.; Escobedo, G. Sucralose: From Sweet Success to Metabolic Controversies—Unraveling the Global Health Implications of a Pervasive Non-Caloric Artificial Sweetener. Life 2024, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- AlDeeb, O.A.A.; Mahgoub, H.; Foda, N.H. Sucralose. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 423–462. ISBN 9780124076914. [Google Scholar]
- Gardner, E. Alternative sugars: Sucralose. Br. Dent. J. 2018, 224, 5. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.N.R.; Román, C.L.; Altamirano, E.M.; Pamatz, F.J.G. Effect of Toothpaste Sweeteners on Glucose Homeostasis. Asian J. Res. Rep. Endocrinol. 2025, 8, 1–9. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by. EFSA J. 2011, 9, 2076. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate and Noncarbohydrate Sweeteners. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–399. [Google Scholar]
- Dümmler, A.; Lawrence, A.M.; de Marco, A. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb. Cell Factories 2005, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, pdb.prot102269. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Urbanowski, M.L.; Greenberg, E.P. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. USA 2004, 101, 15833–15839. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.; Barão, V.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, J.; Li, Z.; Xi, R.; Li, Y.; Peng, X.; Xu, X.; Zheng, X.; Zhou, X. The Effects of Nonnutritive Sweeteners on the Cariogenic Potential of Oral Microbiome. BioMed Res. Int. 2021, 2021, 9967035. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Maetani, M.; Kitada, C.; Nishigami, Y.; Yazawa, A.; Kamitani, S. Analysis of the Effects of Food Additives on Porphyromonas gingivalis. Pathogens 2022, 11, 65. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markus, V. Sucralose Disrupts LuxR-Type Quorum Sensing: Implications for Anti-Cariogenic Activity. Chem. Proc. 2025, 18, 4. https://doi.org/10.3390/ecsoc-29-26692
Markus V. Sucralose Disrupts LuxR-Type Quorum Sensing: Implications for Anti-Cariogenic Activity. Chemistry Proceedings. 2025; 18(1):4. https://doi.org/10.3390/ecsoc-29-26692
Chicago/Turabian StyleMarkus, Victor. 2025. "Sucralose Disrupts LuxR-Type Quorum Sensing: Implications for Anti-Cariogenic Activity" Chemistry Proceedings 18, no. 1: 4. https://doi.org/10.3390/ecsoc-29-26692
APA StyleMarkus, V. (2025). Sucralose Disrupts LuxR-Type Quorum Sensing: Implications for Anti-Cariogenic Activity. Chemistry Proceedings, 18(1), 4. https://doi.org/10.3390/ecsoc-29-26692





