Electrochemical Generation of Aza-BODIPY Polymers as NIR-Absorbing Electrochromic Materials †
Abstract
1. Introduction
2. Materials and Methods
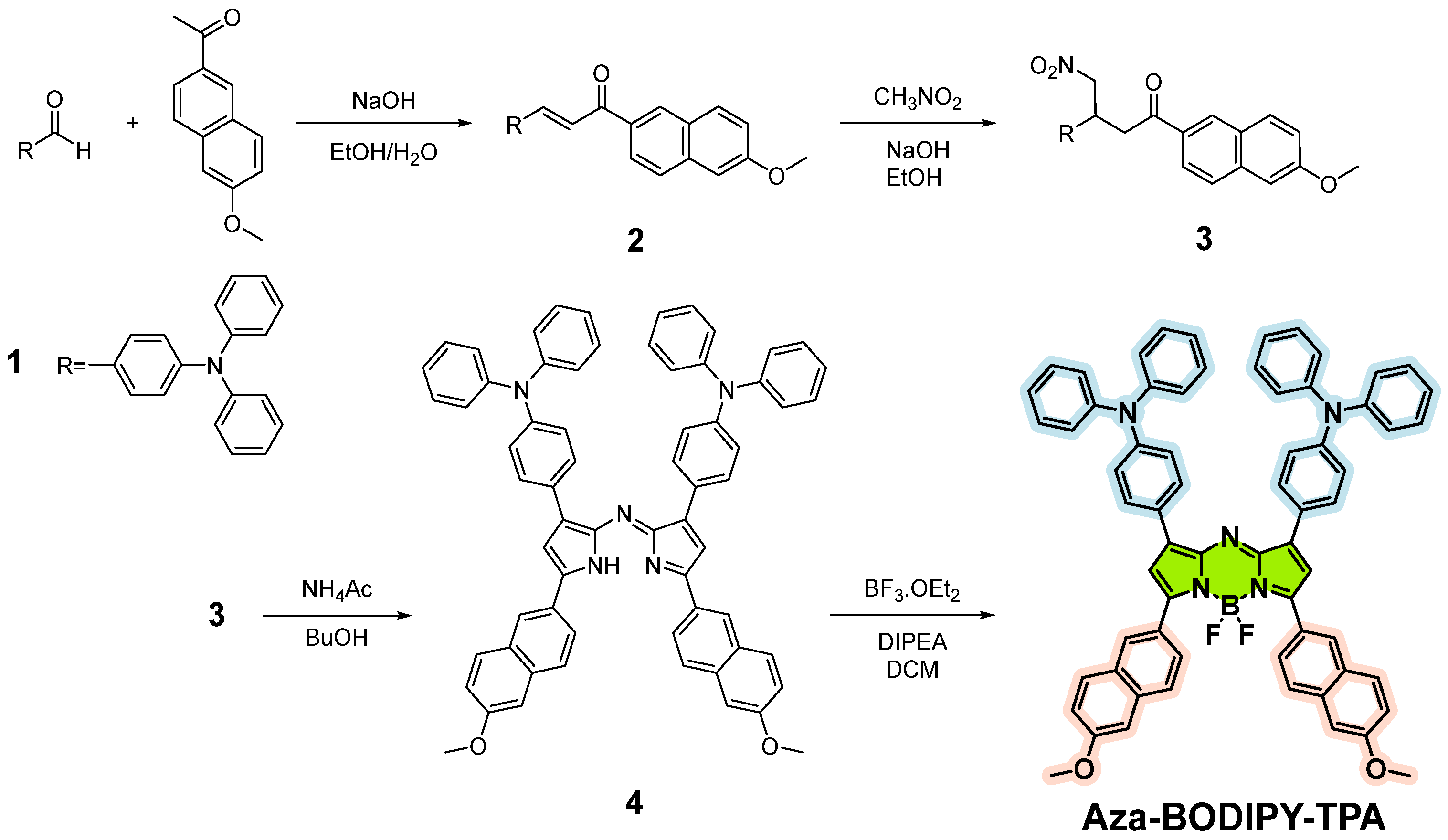
2.1. Monomer Synthesis
2.2. Instrumentation and Methods
3. Results
3.1. Synthesis
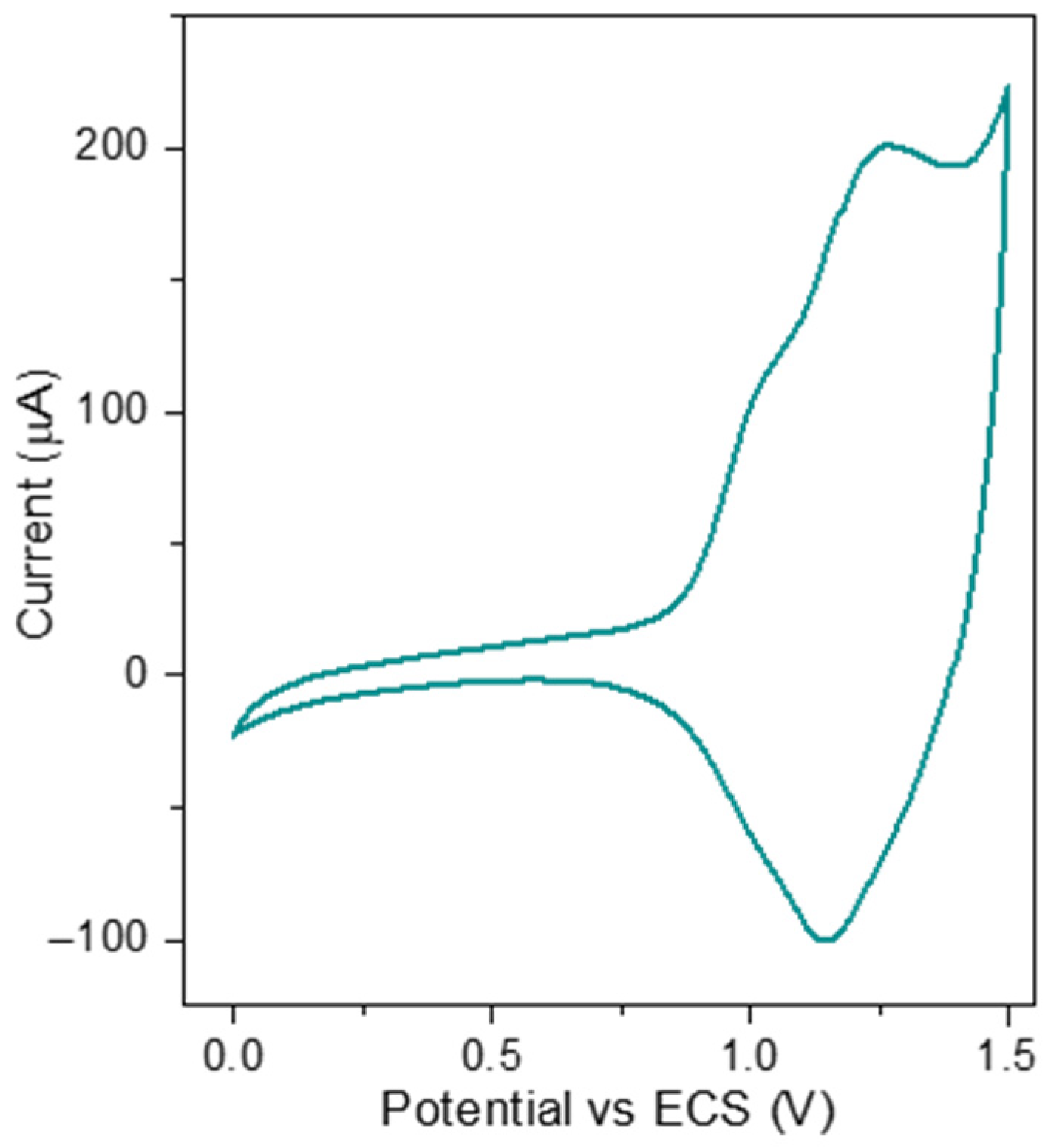
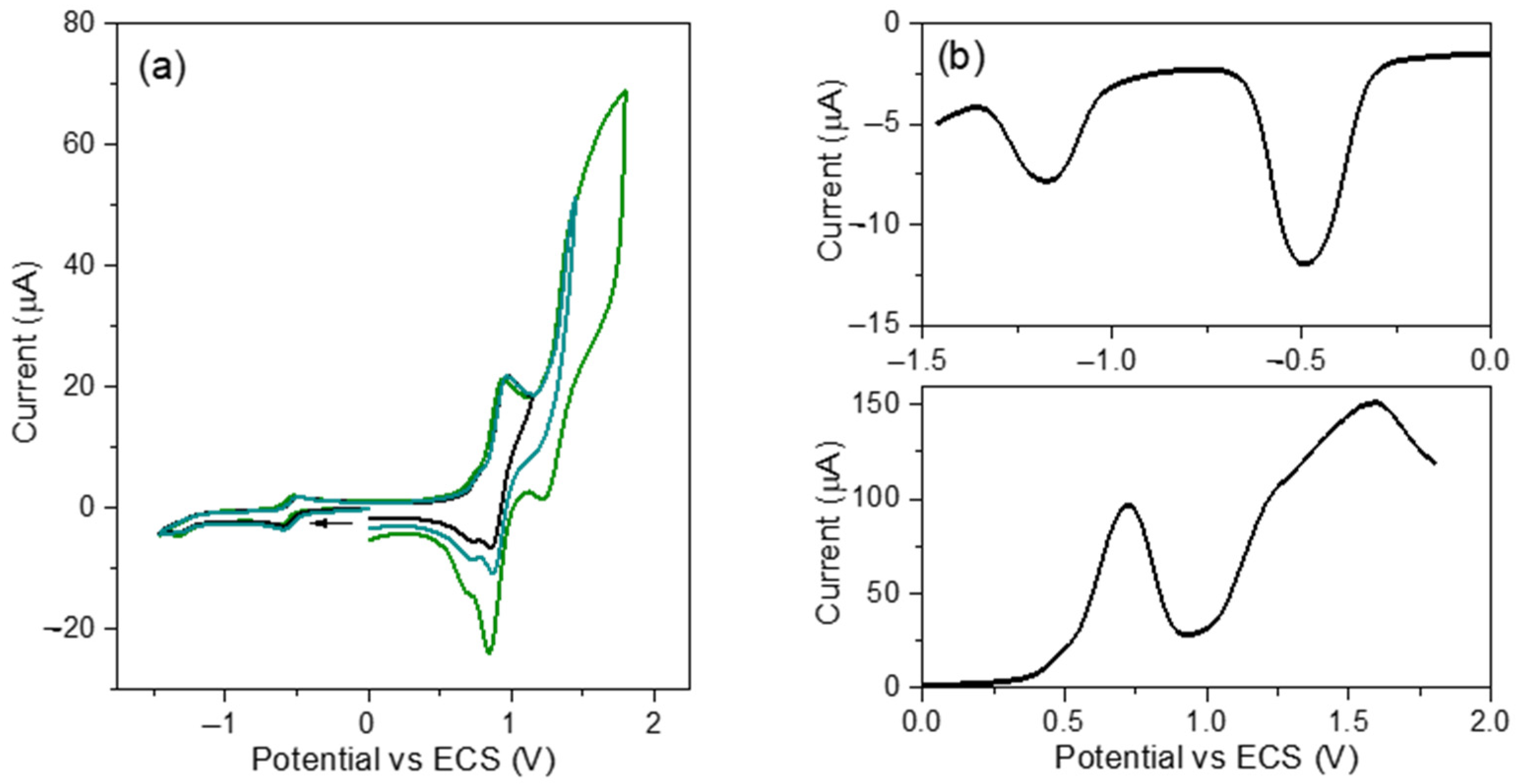
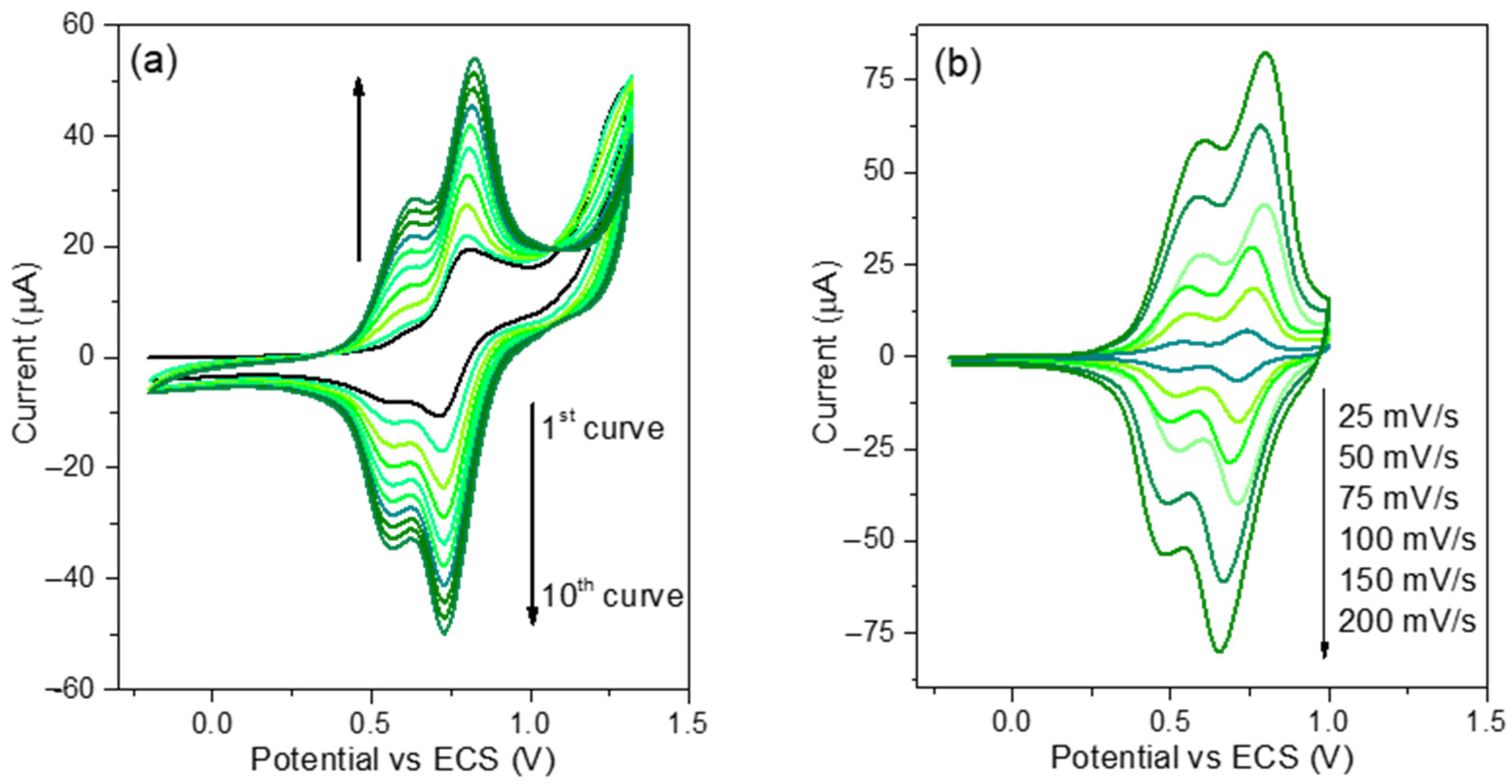
3.2. Electrochemistry
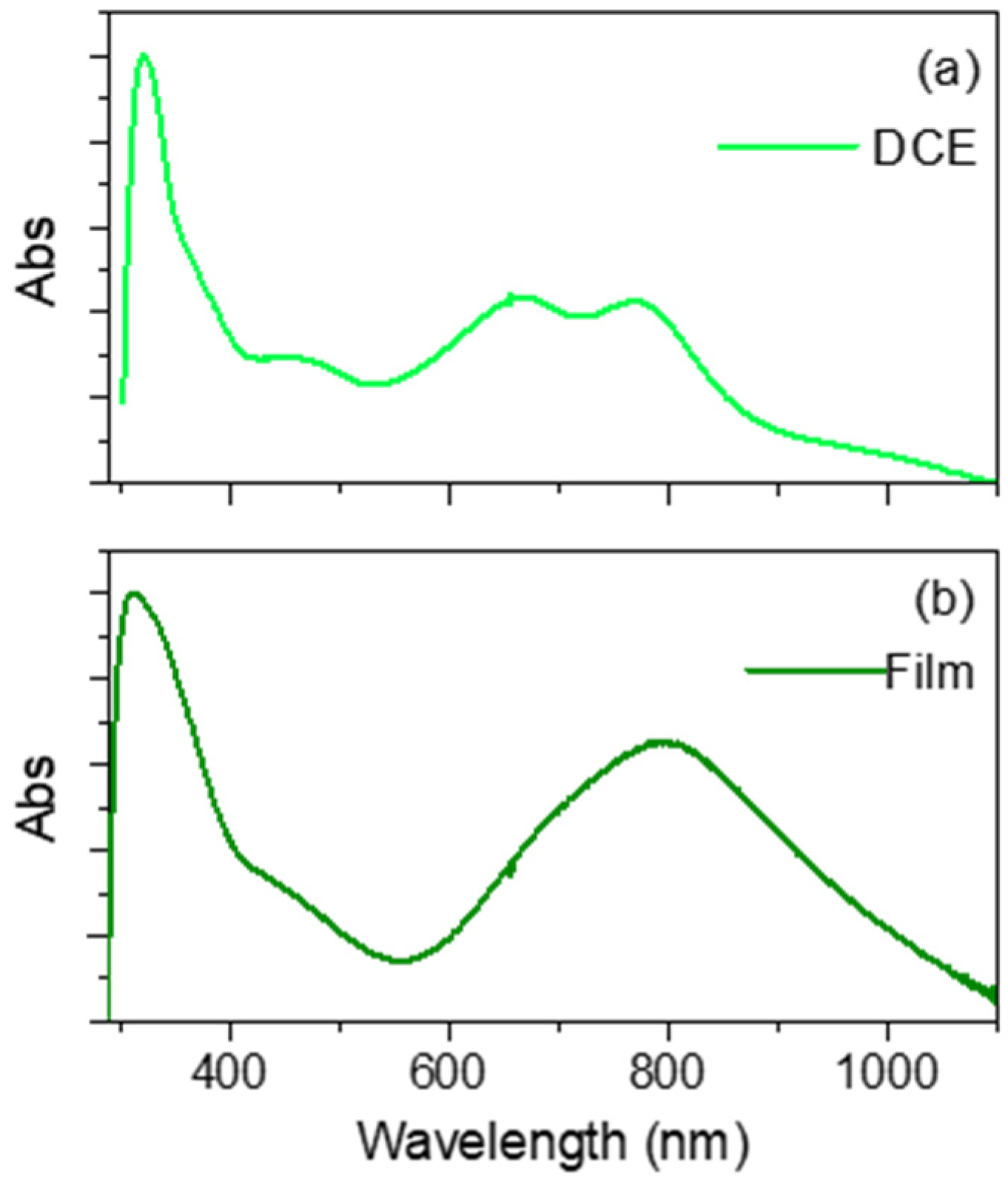
3.3. UV-Visible Absorption Properties of Monomers and Films
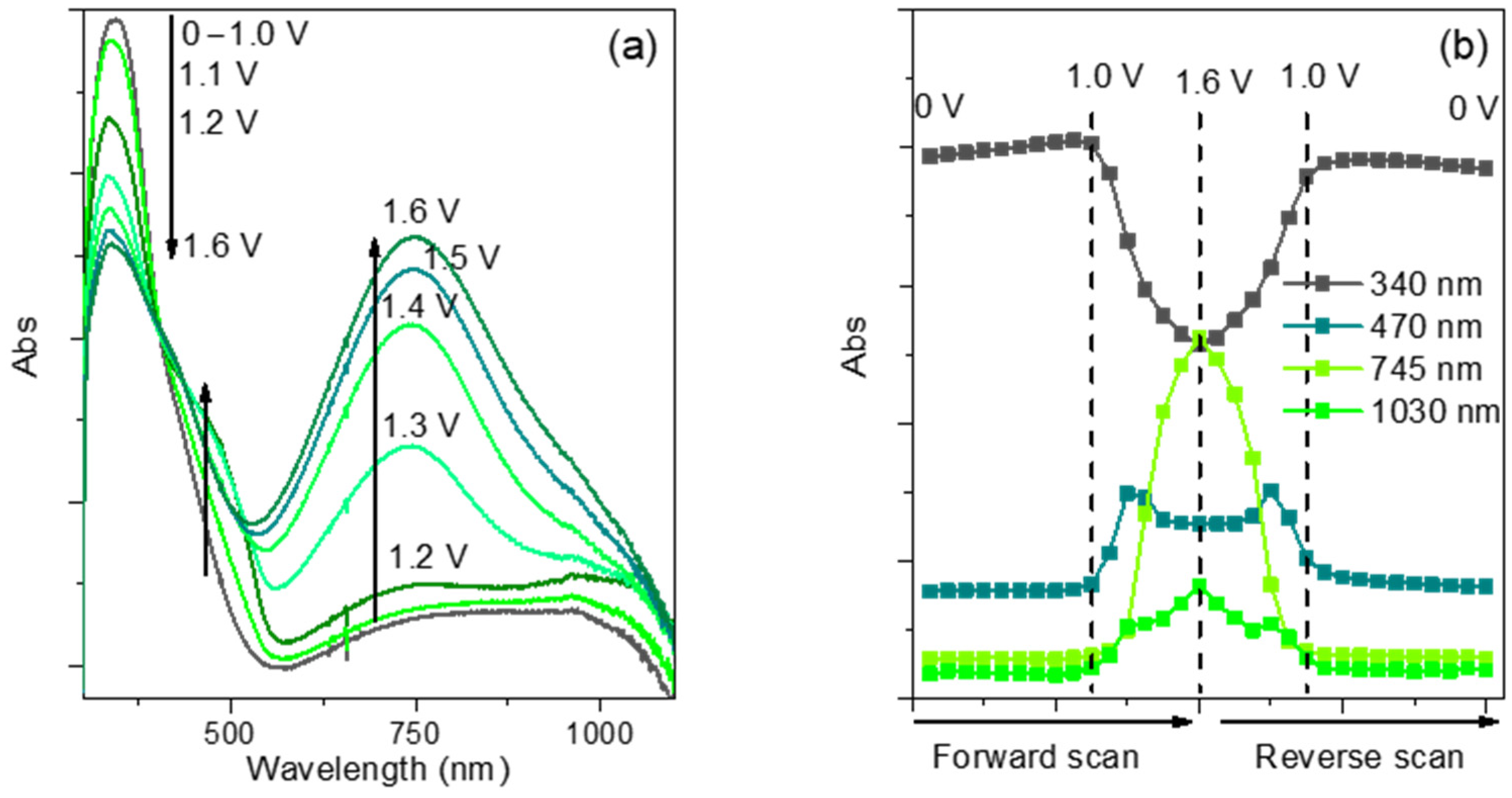
3.4. Spectroelectrochemical Characterization of Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aza-BODIPY | Boron aza-dipyrromethene |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| TPA | Triphenylamine |
Appendix A
References
- Kandpal, S.; Ghosh, T.; Rani, C.; Chaudhary, A.; Park, J.; Lee, P.S.; Kumar, R. Multifunctional electrochromic devices for energy applications. ACS Energy Lett. 2023, 8, 1870–1886. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Abdah, M.A.A.M.; Numan, A.; Moreno-Rangel, A.; Radwan, A.; Khalid, M. Smart window technology and its potential for net-zero buildings: A review. Renew. Sustain. Energy Rev. 2023, 181, 113355. [Google Scholar] [CrossRef]
- Pinjari, D.; Patil, Y.; Misra, R. Near-infrared absorbing aza-BODIPY dyes for optoelectronic applications. Chem. Asian J. 2024, 19, e202400167. [Google Scholar] [CrossRef] [PubMed]
- Heredia, D.A.; Gonzalez Lopez, E.J.; Durantini, E.N.; Durantini, J.; Dittrich, T.; Rappich, J.; Macor, L.; Solis, C.; Morales, G.; Gervaldo, M.; et al. Electrochemical, spectroelectrochemical and surface photovoltage study of ambipolar C60-EDOT and C60-Carbazole based conducting polymers. Electrochim. Acta 2019, 311, 178–191. [Google Scholar] [CrossRef]
- Obłoza, M.; Łapok, Ł.; Pędziński, T.; Stadnicka, K.M.; Nowakowska, M. Synthesis, photophysics and redox properties of aza-BODIPY dyes with electron-donating groups. ChemPhysChem 2019, 20, 2482–2497. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Suarez, M.B.; Perez, M.E.; Heredia, D.A.; Morales, G.M.; Durantini, E.N.; Otero, L.; Gervaldo, M.; Durantini, J.E. Electropolymerized porphyrin films as active materials in organic supercapacitors. A study of the effect of different central metals. Electrochim. Acta 2023, 458, 142552. [Google Scholar] [CrossRef]
- Renfige, M.; Gonzalez Lopez, E.J.; Macor, L.; Solis, C.; Durantini, J.E.; Morales, G.; Otero, L.; Durantini, E.; Heredia, D.; Gervaldo, M. Electrochemical synthesis of donor-acceptor triazine based polymers with halochromic and electrochromic properties. Electrochim. Acta 2024, 486, 144120. [Google Scholar] [CrossRef]
- Yamane, H.; Tanaka, K.; Chujo, Y. Synthesis of a near-infrared light-absorbing polymer based on thiophene-substituted Aza-BODIPY. Polym. J. 2018, 50, 271–275. [Google Scholar] [CrossRef]
- González-Rodríguez, D.; Torres, T. Peripheral functionalization of subphthalocyanines. Eur. J. Org. Chem. 2009, 2009, 1871–1879. [Google Scholar] [CrossRef]
- Lager, E.; Liu, J.; Aguilar-Aguilar, A.; Tang, B.Z.; Pena-Cabrera, E. Novel meso-polyarylamine-BODIPY hybrids: Synthesis and study of their optical properties. J. Org. Chem. 2009, 74, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calosso, A.; López, E.G.; Bermúdez Prieto, E.; Solis, C.; Macor, L.; Durantini, J.; Durantini, E.; Otero, L.; Gervaldo, M.; Heredia, D. Electrochemical Generation of Aza-BODIPY Polymers as NIR-Absorbing Electrochromic Materials. Chem. Proc. 2025, 18, 3. https://doi.org/10.3390/ecsoc-29-26923
Calosso A, López EG, Bermúdez Prieto E, Solis C, Macor L, Durantini J, Durantini E, Otero L, Gervaldo M, Heredia D. Electrochemical Generation of Aza-BODIPY Polymers as NIR-Absorbing Electrochromic Materials. Chemistry Proceedings. 2025; 18(1):3. https://doi.org/10.3390/ecsoc-29-26923
Chicago/Turabian StyleCalosso, Andres, Edwin Gónzalez López, Elizabeth Bermúdez Prieto, Claudia Solis, Lorena Macor, Javier Durantini, Edgardo Durantini, Luis Otero, Miguel Gervaldo, and Daniel Heredia. 2025. "Electrochemical Generation of Aza-BODIPY Polymers as NIR-Absorbing Electrochromic Materials" Chemistry Proceedings 18, no. 1: 3. https://doi.org/10.3390/ecsoc-29-26923
APA StyleCalosso, A., López, E. G., Bermúdez Prieto, E., Solis, C., Macor, L., Durantini, J., Durantini, E., Otero, L., Gervaldo, M., & Heredia, D. (2025). Electrochemical Generation of Aza-BODIPY Polymers as NIR-Absorbing Electrochromic Materials. Chemistry Proceedings, 18(1), 3. https://doi.org/10.3390/ecsoc-29-26923







