Mechanosynthesis of Solid-State Benzoxazoles for Use as OLED †
Abstract
1. Introduction
2. Materials and Methods
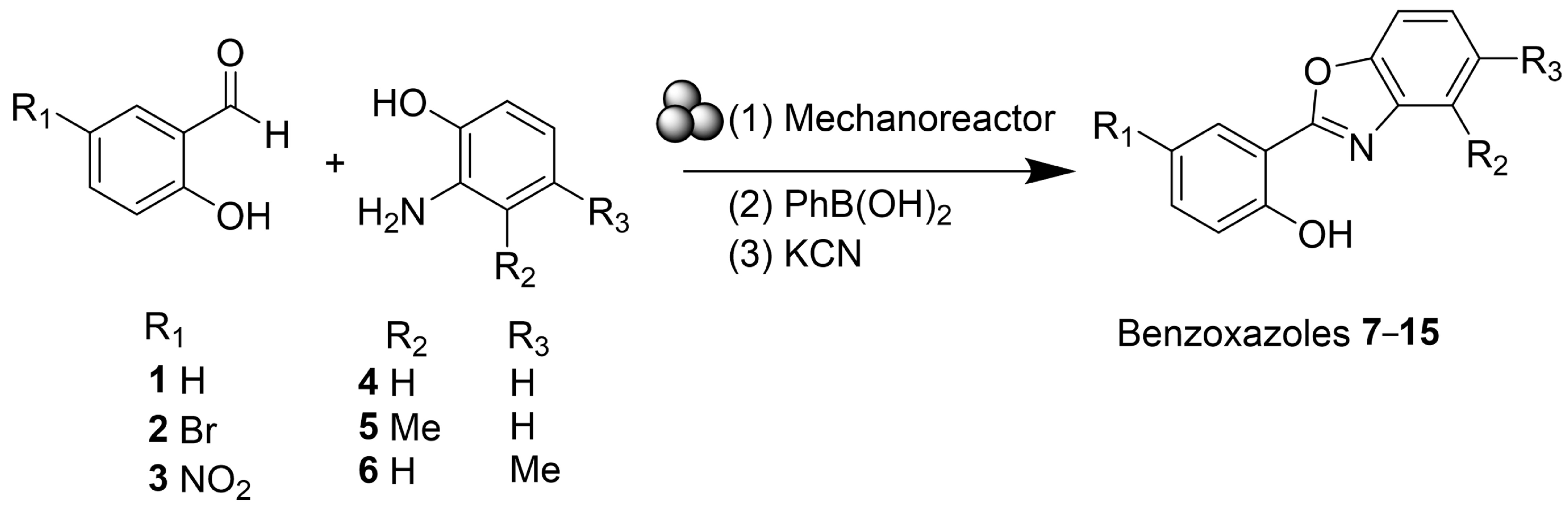
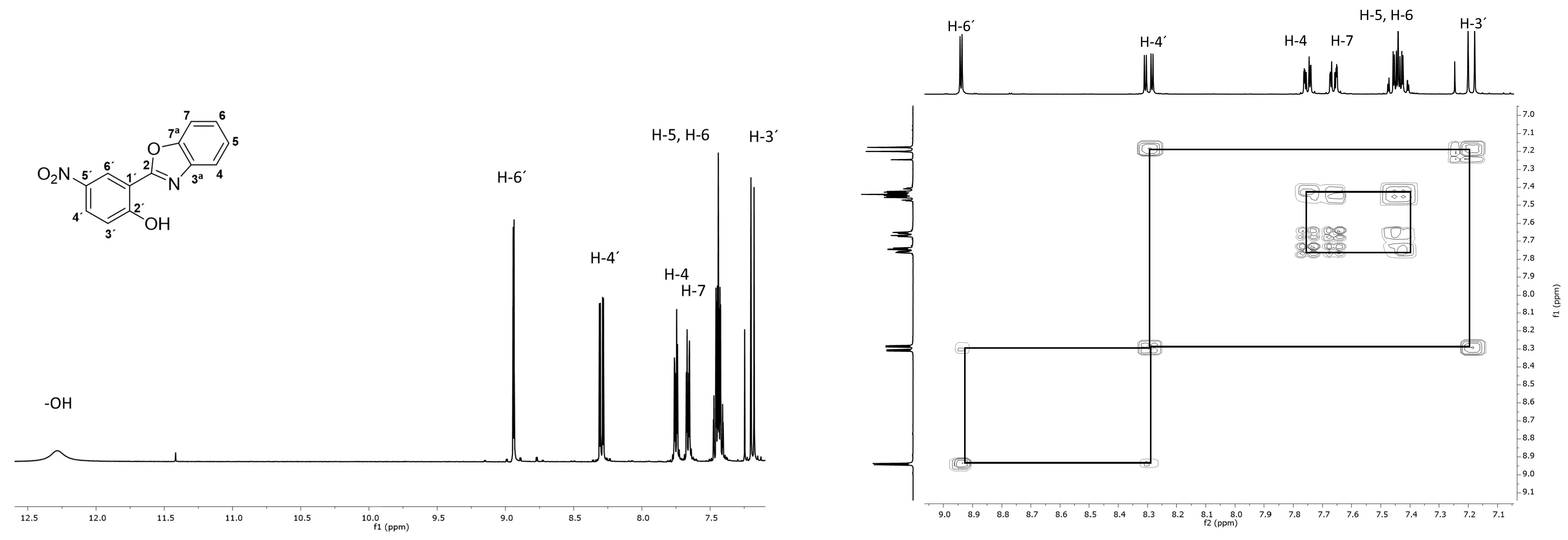
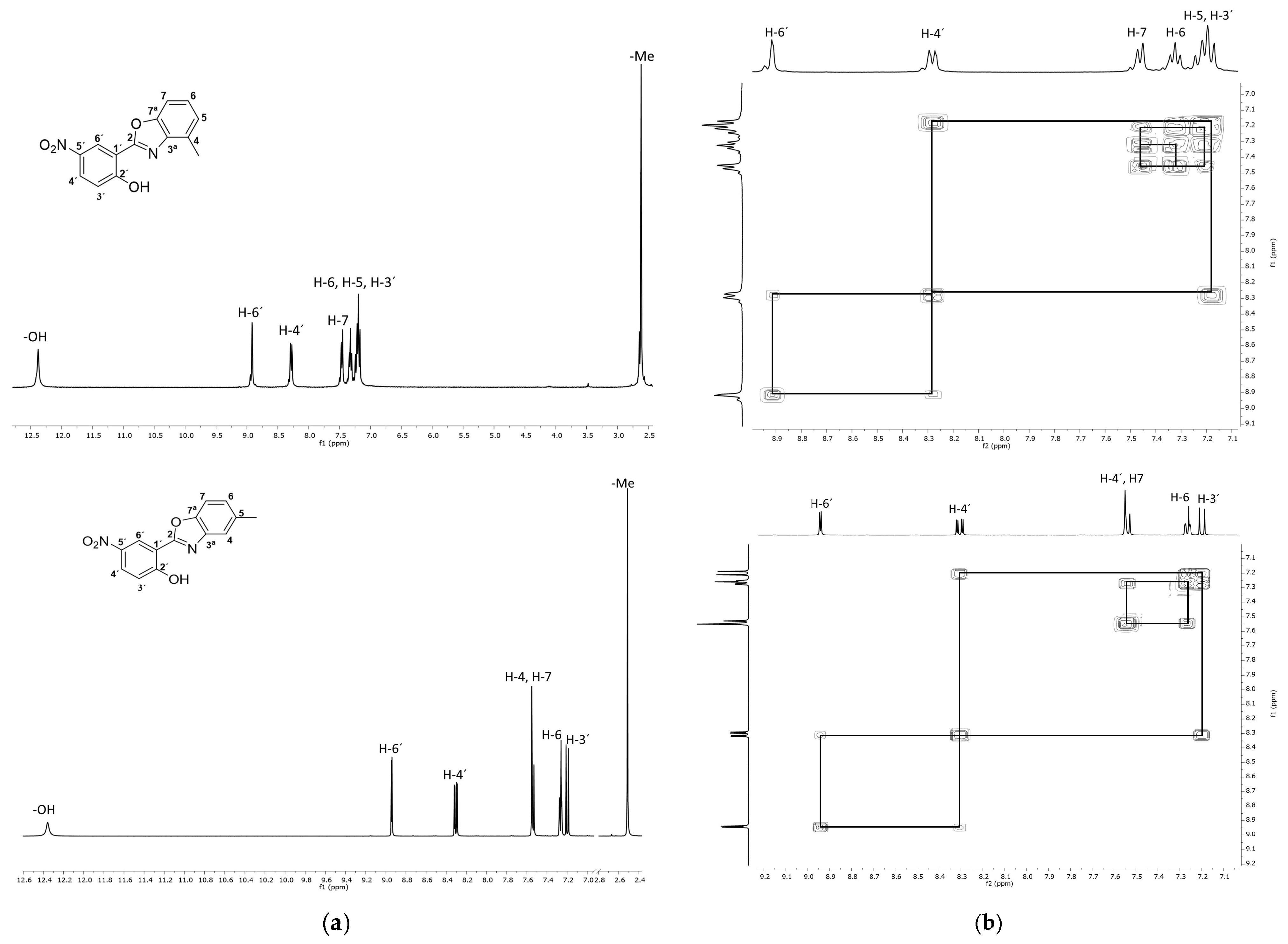
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Templ, J.; Borchardt, L. Mechanochemical strategies applied to the late-stage modifications of pharmaceutically active compounds. Angew. Chem. Int. Ed. 2025, 64, e202503061. [Google Scholar] [CrossRef]
- Wehbe, L.; Bascou, R.; Nesterenko, A.; Guénin, E. Green strategy for sustainable silk lipopeptide production using mechanochemistry. ACS Sustain. Chem. Eng. 2025, 13, 1882–1893. [Google Scholar] [CrossRef]
- Leitão, E.P.T. Comparison of traditional and mechanochemical production processes for nine active pharmaceutical ingredients (APIs). RSC Sustain. 2024, 2, 3655–3668. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Bujňáková, Z. Mechanochemistry in technology: From minerals to nanomaterials and drugs. Chem. Eng. Technol. 2014, 37, 747–756. [Google Scholar] [CrossRef]
- Rivas, M.E. Ball milling towards green synthesis: Applications, projects, challenges. Johns. Matthey Technol. Rev. 2016, 60, 148–150. [Google Scholar] [CrossRef]
- Dong, L.; Li, L.; Chen, H.; Cao, Y.; Lei, H. Mechanochemistry: Fundamental principles and applications. Adv. Sci. 2025, 12, 2403949. [Google Scholar] [CrossRef]
- Losev, E.A.; Zheltikova, D.Y.; Kolybalov, D.S.; Ogienko, A.G.; Boldyreva, E.V. Variation of polymeric material of mechanoreactor walls as a tool for influencing mechanochemical transformations involving molecular crystals. Russ. J. Phys. Chem. A 2025, 99, 1145–1151. [Google Scholar] [CrossRef]
- Schäfer, C.; Török, B. Application of nontraditional activation methods in green and sustainable chemistry: Microwaves, ultrasounds, electro-, photo-, and mechanochemistry, and high hydrostatic pressure. In Nontraditional Activation Methods in Green and Sustainable Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–26. [Google Scholar]
- Ballini, R. Green Synthetic Processes and Procedures; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Tan, D.; Friščić, T. Mechanochemistry for organic chemists: An update. Eur. J. Org. Chem. 2018, 2018, 18–33. [Google Scholar] [CrossRef]
- Bose, A.; Mal, P. Mechanochemistry of supramolecules. Beilstein J. Org. Chem. 2019, 15, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Jiang, C.; Li, Q.; Tan, D.; Bi, S.; Song, J. Performance of OLED under mechanical strain: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 20688–20729. [Google Scholar] [CrossRef]
- Yan, L.; Li, R.; Shen, W.; Qi, Z. Multiple–color AIE coumarin–based Schiff bases and potential application in yellow OLEDs. J. Lumin. 2018, 194, 151–155. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Fleurat-Lessard, P.; Gros, C.P.; Bolze, F.; Xu, H. Synthesis, photophysical properties and two-photon absorption of benzothiazole/benzoxazole π-expanded carbazole dyes. Dye. Pigment. 2022, 204, 110447. [Google Scholar] [CrossRef]
- Bremond, E.; Leygue, N.; Saffon-Merceron, N.; Fery-Forgues, S. 2-Phenylbenzoxazole derivatives as solid-state fluorescence emitters: Influence of steric hindrance and hydrogen bonding on the optical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117586. [Google Scholar] [CrossRef] [PubMed]
- Carayon, C.; Fery-Forgues, S. 2-Phenylbenzoxazole derivatives: A family of robust emitters of solid-state fluorescence. Photochem. Photobiol. Sci. 2017, 16, 1020–1035. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Tupaeva, I.O.; Sayapin, Y.A.; Gusakov, E.A.; Nikolaevskii, S.A.; Demidov, O.P.; Minkin, V.I.; Metelitsa, A.V. Chromogenic properties of 2-(2-carbomethoxy-3,4-dichloro-6-hydroxyphenyl)benzoxazole and its Zn(II) and Cd(II) complexes. Dye. Pigment. 2020, 180, 108417. [Google Scholar] [CrossRef]
- Wang, J.; Baumann, H.; Bi, X.; Shriver, L.P.; Zhang, Z.; Pang, Y. Efficient synthesis of NIR emitting bis [2-(2′-hydroxylphenyl)benzoxazole] derivative and its potential for imaging applications. Bioorganic Chem. 2020, 96, 103585. [Google Scholar] [CrossRef]
- Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623–4631. [Google Scholar] [CrossRef]
- López-Ruiz, H.; Briseño-Ortega, H.; Rojas-Lima, S.; Santillan, R.; Farfán, N. Phenylboronic acid catalyzed-cyanide promoted, one-pot synthesis of 2-(2-hydroxyphenyl)benzoxazole derivatives. Tetrahedron Lett. 2011, 52, 4308–4312. [Google Scholar] [CrossRef]
- Naeimi, H.; Rouzegar, Z.; Rahmatinejad, S. Catalyst-free microwave-promoted one pot synthesis of 2-aryl benzoxazoles using MnO2 nanoparticles as a convenient oxidant under mild condition. Res. Chem. Intermed. 2017, 43, 4745–4758. [Google Scholar] [CrossRef]
- Peña-Zarate, L. Mecanosíntesis de Benzoxazoles Funcionalizados con Potencial Aplicación en el Desarrollo de Diodos Orgánicos Emisores de luz. Ph.D. Thesis, Universidad del Papaloapan, Oaxaca, México, 2020. [Google Scholar]
- Doan, S.H.; Tran, C.B.; Cao, A.L.N.; Le, N.T.H.; Phan, N.T.S. A new pathway to 2-arylbenzoxazoles and 2-arylbenzothiazoles via one-pot oxidative cyclization reactions under iron-organic framework catalysis. Catal. Lett. 2019, 149, 2053–2063. [Google Scholar] [CrossRef]
- Henry, M.C.; Abbinante, V.M.; Sutherland, A. Iron-catalyzed regioselective synthesis of 2-arylbenzoxazoles and 2-arylbenzothiazoles via alternative reaction pathways. Eur. J. Org. Chem. 2020, 19, 2819–2826. [Google Scholar] [CrossRef]

| Benzoxazole | Yield (%) | Reaction Time (min) | Imine | Yield (%) | Reaction Time (min) |
|---|---|---|---|---|---|
| 7 | 56.15 | 70 | 16 | 91.22 | 2 |
| 8 | 25.12 | 70 | 17 | 96.35 | 2 |
| 9 | 52.45 | 70 | 18 | 88.19 | 2 |
| 10 | 53.03 | 70 | 19 | 88.85 | 2 |
| 11 | 24.41 | 180 | 201 | 42.50 | 20 |
| 12 | 40.61 | 120 | 21 | 97.56 | 4 |
| 13 | 36.98 | 120 | 22 | 92.04 | 4 |
| 14 | 18.76 | 180 | 231 | 34.12 | 20 |
| 15 | 24.30 | 120 | 24 | 89.31 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Galero, N.X.; Peña-Zarate, L. Mechanosynthesis of Solid-State Benzoxazoles for Use as OLED. Chem. Proc. 2025, 18, 13. https://doi.org/10.3390/ecsoc-29-26667
Martínez-Galero NX, Peña-Zarate L. Mechanosynthesis of Solid-State Benzoxazoles for Use as OLED. Chemistry Proceedings. 2025; 18(1):13. https://doi.org/10.3390/ecsoc-29-26667
Chicago/Turabian StyleMartínez-Galero, Nelda Xanath, and Lucio Peña-Zarate. 2025. "Mechanosynthesis of Solid-State Benzoxazoles for Use as OLED" Chemistry Proceedings 18, no. 1: 13. https://doi.org/10.3390/ecsoc-29-26667
APA StyleMartínez-Galero, N. X., & Peña-Zarate, L. (2025). Mechanosynthesis of Solid-State Benzoxazoles for Use as OLED. Chemistry Proceedings, 18(1), 13. https://doi.org/10.3390/ecsoc-29-26667







