One-Pot Synthesis of Imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé Reaction †
Abstract
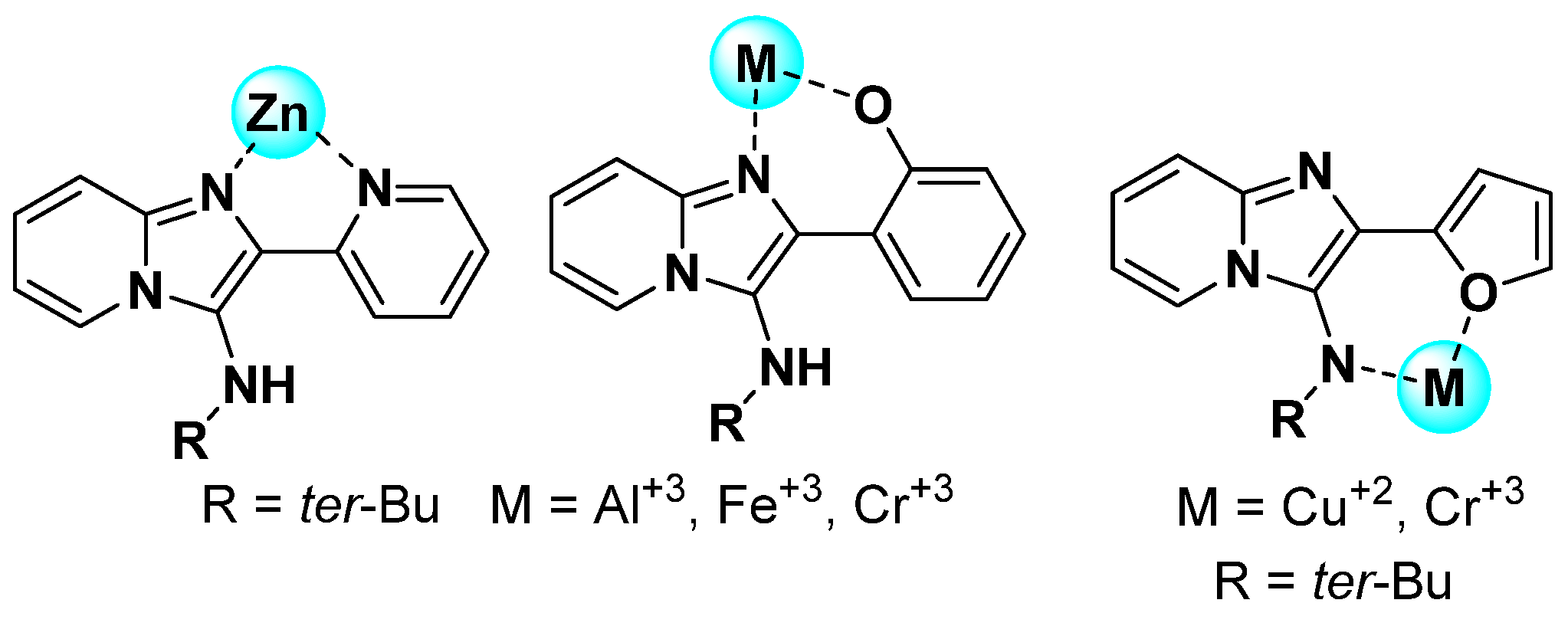
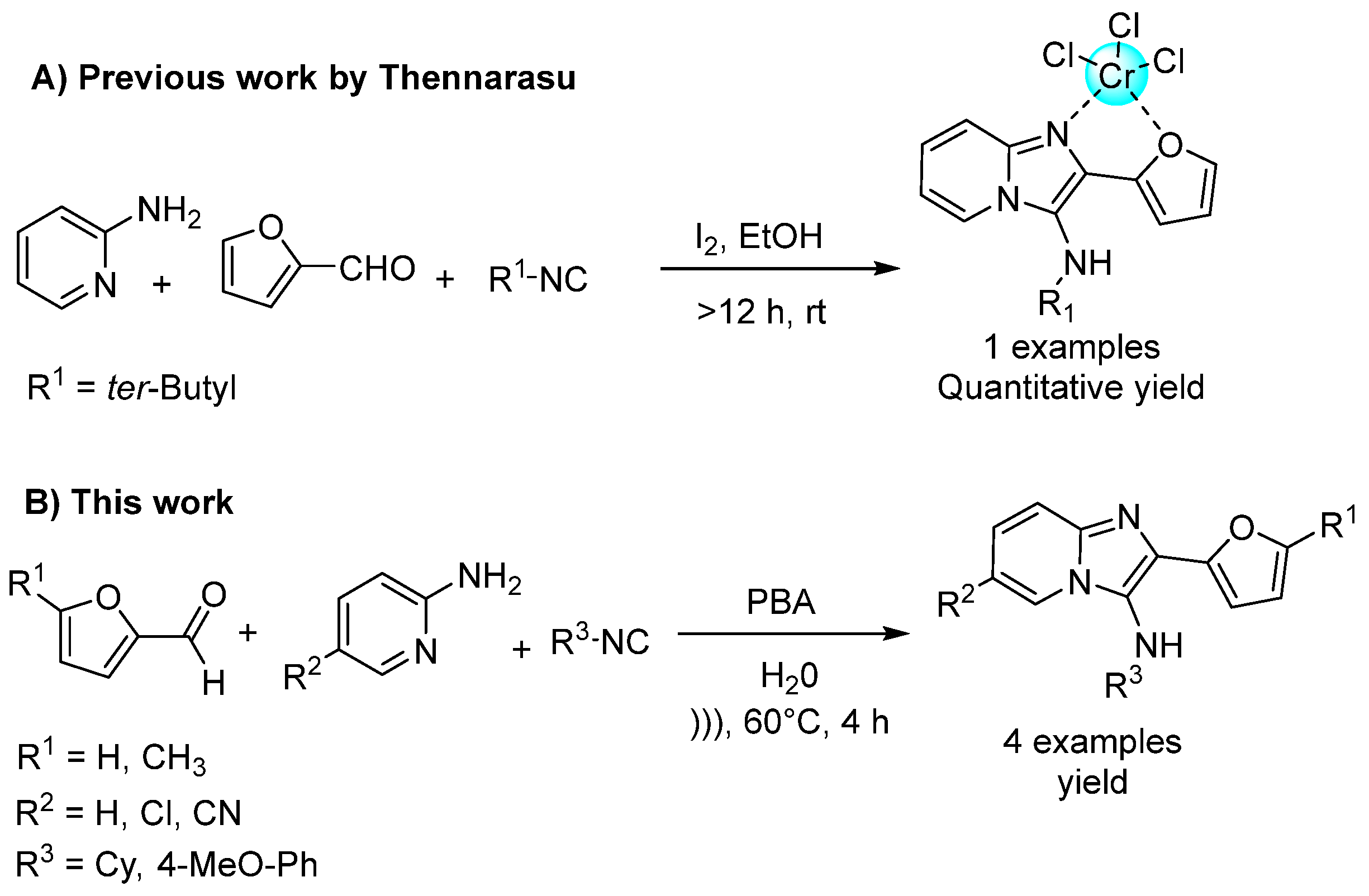
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information, Instrumentation, and Chemicals
3.2. General Procedure (GP)
3.3. Spectral Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammad Abu-Taweel, G.; Alharthi, S.S.; Al-Saidi, H.M.; Babalghith, A.O.; Ibrahim, M.M.; Khan, S. Heterocyclic Organic Compounds as a Fluorescent Chemosensor for Cell Imaging Applications: A Review. Crit. Rev. Anal. Chem. 2024, 54, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2022, 54, 2148–2165. [Google Scholar] [CrossRef] [PubMed]
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Raiguru, B.P.; Mishra, M.; Mohapatra, S.; Nayak, S. Recent Advances in the Synthesis of Imidazo[1,2-a]Pyridines: A Brief Review. ChemistrySelect 2022, 7, e202103987. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Wang, M.X. Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–471. [Google Scholar]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B. Recent Developments on Ultrasound-Assisted One-Pot Multicomponent Synthesis of Biologically Relevant Heterocycles. Ultrason. Sonochem. 2017, 35, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Kurva, M.; Claudio-Catalán, M.Á.; Rentería-Gómez, Á.; Jiménez-Halla, J.O.C.; González-García, G.; Velusamy, J.; Ramos-Ortíz, G.; Castaño-González, K.; Piazza, V.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of Luminescent Imidazo[1,2-a]Pyridine-3-Amines. Studies of Fluorescence, Solvatochromism, TD-DFT Calculations and Bioimaging Application. J. Mol. Struct. 2023, 1276, 134797. [Google Scholar] [CrossRef]
- Basavanag, U.M.V.; Islas-Jácome, A.; Rentería-Gómez, A.; Conejo, A.S.; Kurva, M.; Jiménez-Halla, J.O.C.; Velusamy, J.; Ramos-Ortíz, G.; Gámez-Montaño, R. Synthesis of 2-Julolidin-Imidazo[1,2-a]Pyridines via Groebke–Blackburn–Bienaymé Reaction and Studies of Optical Properties. New J. Chem. 2017, 41, 3450–3459. [Google Scholar] [CrossRef]
- Divya, D.; Ramanjaneyulu, M.; Nandhagopal, M.; Srinivasan, V.; Thennarasu, S. A Fluorescent Chemosensor for Selective Detection of Chromium (III) Ions in Environmentally and Biologically Relevant Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 316, 124286. [Google Scholar] [CrossRef] [PubMed]

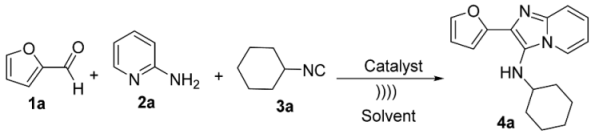 | ||||
|---|---|---|---|---|
| Entry [a] | Catalyst [b] | Solvent [1.0 M] | Time (h) | Yield [e] |
| 1 | --- | --- | 4 | --- |
| 2 | --- | H2O | 4 | traces |
| 3 [c] | NH4Cl | H2O | 4 | 32 |
| 4 | NH4Cl | H2O | 4 | 50 |
| 5 | PBA | H2O | 4 | 65 |
| 6 [d] | PBA | H2O | 4 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Rangel, D.; Corona-Díaz, A.; González-Gámez, I.A.; Gámez-Montaño, R. One-Pot Synthesis of Imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé Reaction. Chem. Proc. 2025, 18, 10. https://doi.org/10.3390/ecsoc-29-26678
Calderón-Rangel D, Corona-Díaz A, González-Gámez IA, Gámez-Montaño R. One-Pot Synthesis of Imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé Reaction. Chemistry Proceedings. 2025; 18(1):10. https://doi.org/10.3390/ecsoc-29-26678
Chicago/Turabian StyleCalderón-Rangel, David, Alejandro Corona-Díaz, Indhira A. González-Gámez, and Rocío Gámez-Montaño. 2025. "One-Pot Synthesis of Imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé Reaction" Chemistry Proceedings 18, no. 1: 10. https://doi.org/10.3390/ecsoc-29-26678
APA StyleCalderón-Rangel, D., Corona-Díaz, A., González-Gámez, I. A., & Gámez-Montaño, R. (2025). One-Pot Synthesis of Imidazo[1,2-a]pyridines via Groebke–Blackburn–Bienaymé Reaction. Chemistry Proceedings, 18(1), 10. https://doi.org/10.3390/ecsoc-29-26678






