1. Introduction
Increasing multidrug-resistance to bacterial pathogens represents a challenging issue: due to the lack of an innovative research and development of novel antibiotics, the burden of untreatable infections is projected to increase at immense economic costs in the coming years [
1]. More than 8000 fungi species are known to cause diseases in plants, with devastating pathogens of commercial significance that include oomycetes from the genus
Phytophthora that lead to significant economic losses from reduction in orchard health and yield [
2]. The detrimental impact extends to human organisms: the emergence and global spread of highly contagious pathogenic fungi, such as the multidrug-resistant
Candida auris and azole-resistant
Aspergillus, present a significant and escalating threat to global health [
3,
4,
5,
6]. The growing resistance to front-line therapies like azoles and echinocandins, combined with the high toxicity, carcinogenicity and environmental concerns of many fungicides create an urgent need to develop new antifungal therapeutics with novel modes of action to circumvent existing resistance mechanisms [
7].
Alongside organic molecules, metal-based compounds represent a rich and underexplored source of novel antimicrobial agents. For many decades, copper ions have been used as antibacterial agents, pesticides, and fungicides. Copper-based antimicrobial compounds, like the Bordeaux mixture, have been extensively used to protect crops against diseases caused by bacteria, fungi, and oomycetes and remain effective even against some copper-tolerant pathogens. Copper salts have been used for the treatment of mildew in grapes. However, the use of copper ions presents several disadvantages, such as hydrophilicity, low stability, and high concentrations of copper salts necessary for formulations [
8]. Therefore, the strategy of copper(II) complexation by organic ligands is currently widely employed for the development of antifungal compounds. It has often been observed that metal complexes outperform their respective parent ligands in inhibition of bacterial and fungal growth [
9].
Antibacterial activity of catechol derivatives is commonly associated with inactivation of bacterial enzymes, as well as with the disruption of bacterial energy production resulting from the inhibition of oxidative phosphorylation and uncoupling of electron transport from ATP synthesis [
10]. Oxidized forms of catechols, e.g., semiquinone radicals and
o-benzoquinones, interact with sulfhydryl groups of proteins and glutathione, inducing protein cross-linking and oxidative stress [
11]. Since chelation reduces the polarity of the metal ion and favors charge distribution among the ligand molecules, lipophilicity of the system increases, facilitating its permeation through the lipid layers of the cell membrane and thereby enhancing the antibacterial effect [
12]. Copper(II) complexes of catechol derivatives exhibit a potent antibacterial activity primarily through ROS generation in Fenton-like reactions, which leads to oxidative stress and direct cellular damage, including DNA and RNA cleavage, protein fragmentation, and lipid peroxidation [
13]. Copper(II) complexes further exert antibacterial effect by displacing iron from solvent-exposed [4Fe-4S] clusters in proteins, elevating intracellular iron levels and amplifying radical formation [
14]. It should also be noted that genotoxicity of copper(II) compounds is manifested through specific binding to guanine bases in DNA and direct causation of strand breaks. Furthermore, in the presence of copper(II) ions, catechol derivatives may enzymatically oxidize with the formation of superoxide anion radicals that are subsequently reduced to hydrogen peroxide and hydroxyl radicals. Such a synergetic effect is known to induce DNA strand breaks, chromosome aberrations, and chromatid exchanges in the bacterial cell [
10].
For the development of antifungal agents, thiosemicarbazones represent a promising class of compounds, whose antifungal properties have long been explained by their ability to form complexes with copper(II) ions essential to the functioning of fungal cells [
15]. This hypothesis is strongly supported by the markedly poor activity of their oxygen analogs, hydrazones, which have a markedly lower ability to form such complexes [
15]. Furthermore, the presence of a hydrogen atom, which allows for the tautomerism crucial for metal complexation, on the respective nitrogen atom is reported to be an essential requirement for antifungal properties: N
2-methyl derivatives generally display little to no antifungal activity. Further mechanisms of antifungal action proposed for thiosemicarbazones include inhibition of ergosterol synthesis, interaction with fungal membrane sterols, and inhibition of macromolecular synthesis [
16,
17]. However, metal complexes offer a distinct advantage over purely organic compounds due to the chelation effect, which increases lipophilicity and favors permeation through the fungal cell wall, and completely different modes of action, primarily ROS generation leading to the oxidation of the components of the fungal cell wall, such as glucan and chitin, causing irreversible damage and disintegration [
9,
18]. Additionally, copper(II) complexes may directly target and cleave the cellular DNA, causing single and double-strand breaks in plasmid DNA (e.g., pBR322), converting its supercoiled form into nicked and linear forms. As another mechanism of the biocidal action that copper(II) compounds exert on fungal cells, it should be noted that copper represents an essential trace element in fungi that coordinates with histidine, glutamic acid, aspartic acid, methionine and cysteine amino acid residues and hence maintains the proper functioning of essential enzymes, e.g., COX, amine oxidase, ETRs, tyrosinase, phenol oxidase, Cu/Zn SOD, lysyl oxidase and laccase [
19]. Therefore, coordination to amino acids and ligand displacement that result in enzyme dysregulation appear to be a plausible mechanism of antifungal activity of copper(II) complexes as well.
In the present work, we have synthesized 4,6-di-tert-butyl-2,3-dihydroxybenzaldehyde-derived hydrazone (compound 1) and thiosemicarbazone (compound 2) and their copper(II) complexes (compounds 1a, 2a, respectively). This manuscript explores the antibacterial and antifungal potential of the synthesized hydrazones and thiosemicarbazones and their copper(II) complexes, discussing their structure-activity relationships and mechanisms of action in detail, with a specific focus on their application against challenging pathogens like Gram-positive (Bacillus subtilis, Staphylococcus saprophyticus) and Gram-negative (Escherichia coli, Pseudomonas putida) bacteria, as well as an oomycete species Phytophthora infestans. The obtained data could provide valuable insights for the pharmaceutical design and development of novel antibacterial and antifungal agents, as well as for the elucidation of their mechanisms of biocidal action.
2. Materials and Methods
Copper was determined using an atomic emission spectrometer with an inductively coupled plasma excitation source (Spectroflame Modula, Kleve, Germany). 1H NMR spectra were recorded at 500 MHz with a Bruker Avance-500 spectrometer (Bruker, Karlsruhe, Germany). ESI-MS spectra were registered using a Shimadzu LC-MS 2020 spectrometer (Shimadzu, Kyoto, Japan) using direct injection of the specimens into the ion source. UV-Vis absorption spectra of the compounds were recorded in acetonitrile (HPLC grade) using a Solar PB 2201 spectrophotometer (Solar, Minsk, Belarus) using a quartz cuvette with a 1 cm optical path. FT-IR spectra were recorded in the wavelength range of 400–4000 cm−1 with an AVATAR FT-IR-330 (Thermo Nicolet) spectrometer (Thermo Fisher Scientific, Waltham, USA) using a Smart Diffuse Reflectance accessory (Thermo Fisher Scientific, Waltham, USA). Molar conductance of 10−3 M solutions of the copper(II) complexes in acetonitrile was measured at room temperature using a TESLA BMS91 conductometer (Metrohm, Herisau, Switzerland, cell constant 1.0). Thermogravimetric analysis was performed using an STA449-F3 Jupiter synchronous thermal analyser (Netzsch, Selb, Germany) at a heating rate of 10 K · min−1 in the temperature range from 30 to 880 °C in a nitrogen atmosphere using an Al2O3 crucible. X-ray diffractograms were recorded at room temperature with CuKα monochromated radiation in the range of 2θ = 7–90° using a DRON-2.0 diffractometer (Bourevestnik, Saint Petersburg, Russia). ESR spectra of the copper(II) complexes were recorded with an ERS-220 spectrophotometer, X-band (9.45 GHz).
Escherichia coli, Pseudomonas putida, Bacillus subtilis, and Staphylococcus saprophyticus strains were obtained from the collection of the Department of Molecular Biology of the Belarusian State University. Microorganisms were subcultured for testing in LB medium (pH 7.4 ± 0.2). The cells were suspended in saline according to the McFarland protocol to prepare a suspension of approximately 106 CFU · mL−1. Stock solutions of the compounds (1.6 mg · mL−1) in DMSO were diluted to the concentrations ranging from 400 to 10 mg · mL−1, and a 24 h-old inoculum was added to each tube. The final concentration of DMSO (1–2%) in the medium had no effect on the growth of the test organisms. Incubation was carried out at optimal growth temperature for 20 h, and the optical density (OD600) was measured for bacterial cultures with and without the test compound. MIC, defined as the lowest concentration of the test compound to inhibit the visible growth of microbes, was determined after an incubation period. A parallel study was performed using DMSO as a negative control. All results were checked in three separate experiments. Tetracycline and streptomycin served as positive controls for bacteria.
In the antifungal activity tests, the mycelium of Phytophtora infestans was inoculated as agar blocks onto Petri dishes containing Rye A medium and cultured at 18 °C for 2–3 weeks. The copper(II) complexes were dissolved in 100% DMSO at the concentration of 10 mg/mL, and the resulting solution was added to the Petri dishes to achieve the desired final concentrations of 50 µg/mL and 100 µg/mL. The final volume of DMSO in all dishes was 1%. 10 ml of Rye A medium was added to each Petri dish. After the dishes were dried, the P. infestans mycelium was inoculated as blocks of equal diameter (1 cm × 1 cm) onto the Petri dishes. DMSO was added to the control dishes to achieve the final concentration of 1%. The dishes were incubated for 2 weeks at the temperature of 18 °C. The diameter of the grown mycelium zone was measured, and the mycelium growth area was calculated based on the measured diameter.
3. Results
Synthesis of the ligands
1,
2. Methodology of the synthesis of the two compounds is presented in [
20].
Compound 1. Light-yellow powder, yield 67%, M.p. 265–268 °C. 1H NMR (500 MHz, DMSO-d6): 1.36 s (9H, 3CH3), 1.42 s (9H, 3CH3), 6.79 s (1H, CHarom), 7.85–7.86 m (2H, CHpyridine), 8.42 s (1H, OH), 8.82–8.83 m (2H, CHpyridine), 9.36 s (1H, CH=N), 12.45 s (1H, NH), 13.06 s (1H, OH). 13C NMR (126 MHz, DMSO-d6): 29.13, 32.53, 34.90, 35.18, 112.25, 114.14, 121.52, 136.07, 138.68, 139.80, 142.26, 148.37, 150.49, 150.79, 161.21. FT-IR (ν, cm−1): 3505 s ν(O–H); 3433 s ν(N–H); 1667 s ν(C=O); 1620, 1598 s ν(C=N); 1169, 1101, 1066 s ν(C–O). λ, nm (lgε, M−1cm−1): 205, 221 (3,67), 320 (3,73). EI-MS, m/z (Irel, %): 369.20 (80) [M]+. [C21H27O3N3]+.
Compound 2. Light-yellow powder, yield 79%, M.p. 256–258 °C. 1H NMR (500 MHz, DMSO-d6): 1.35 s (9H, 3CH3), 1.36 s (9H, 3CH3), 6.78 s (1H, CHapom), 7.95 br. s (1H), 8.17 br. s (1H), 8.23 s (1H, OH), 8.93 s (1H, CH=N), 10.33 br. s (1H), 11.60 s (1H, OH). FT-IR (ν, cm−1): 3533, 3505 s ν(O–H); 3134 s ν(N–H); 1615, 1589 s ν(C=N); 1207 s ν(C=S); 1171, 1120, 1086 s ν(C–O). λ, nm (lgε, M−1cm−1): 207, 328 (3.60). EI-MS, m/z (Irel, %): 323.15 (92) [M]+. [C16H25N3O2S]+.
Synthesis of the copper(II) complexes 1a, 2a. A solution of copper(II) acetate in methanol was added dropwise to a solution of the ligand in chloroform (CH3OH:CHCl3 = 1:3 (v/v)) at the copper:ligand molar ratio of 1:1. The solution was stirred on a magnetic stirrer for 30 min, and the formed precipitate was filtered off, washed with methanol, recrystallized from ethanol and dried at room temperature.
Results of the antibacterial and antifungal assays for the compounds
1,
1a,
2,
2a are presented in
Table 1,
Table 2 and
Table 3.
Compound 1a. Dark-brown solid, yield 89%. Anal. calcd for C42H50Cu2N6O6: C, 58.52; H, 5.85; Cu, 14.74; N, 9.75; O, 11.14; found: C, 58.76; H, 5.83; Cu, 14.80; N, 9.71; O, 11.09. Molar conductivity Ωm: 7.50. FT-IR (ν, cm−1): 3398 m ν(O–H); 1591 s ν(C=N); 1380 s, 1234 s ν(C–O); 636 w, 596 w ν(Cu–O); 480 w ν(Cu–N). UV-Vis: λ, nm (logε, M−1‧cm−1): 213, 243 (4.57), 384 (4.46), 452sh. ESR data: ESI-MS (+) calcd for [C21H25CuN3NaO3]+ 453.11, found 453.15.
Compound 2a. Light-brown solid, yield 84%. Anal. calcd for C32H46Cu2N6O4S2: C, 49.92; H, 6.02; Cu, 16.51; N, 10.91; O, 8.31; S, 8.33; found: C, 49.72; H, 6.04; Cu, 16.58; N, 10.95; O, 8.34; S, 8.36. Molar conductivity Ωm: 4.32. FT-IR (ν, cm−1): 3491 s ν(O–H); 1593 s ν(C=N); 1225 s, 1171 s ν(C–O); 596 w ν(Cu–O); 536 w ν(Cu–N); 430 w ν(Cu–S). UV-Vis: λ, nm (logε, M−1‧cm−1): 214, 244sh, 337 (4.46), 407sh, 571sh. ESR data: = 2.208; = 2.056. ESI-MS (+) calcd for [C17H29CuN3O3S]+ 418.12, found 418.95.
4. Discussion
The formation of copper(II) complexes with hydrazone (compound
1) and thiosemicarbazone (compound
2) ligands has been verified by physicochemical analysis methods. FT-IR spectra of the copper(II) complexes
1a,
2a display a hypsochromic shift in the absorption bands corresponding to valent vibrations of azomethine and hydroxyl groups compared to those appearing in the FT-IR spectra of the parent ligands. Compared to the ligands
1,
2, a shift in the absorption bands corresponding to the C–O bond valent vibrations is observed to the lower frequency region in their copper(II) complexes
1a,
2a. Coordination of ligands to the copper(II) ion in the enol form rather than in the form of a keto tautomer is confirmed by the disappearance of absorption bands corresponding to valent vibrations of C=O and N-H groups in the FT-IR spectra of their copper(II) complexes [
21]. Furthermore, new absorption bands at 596 cm
−1, 480–536 cm
−1 and 430 cm
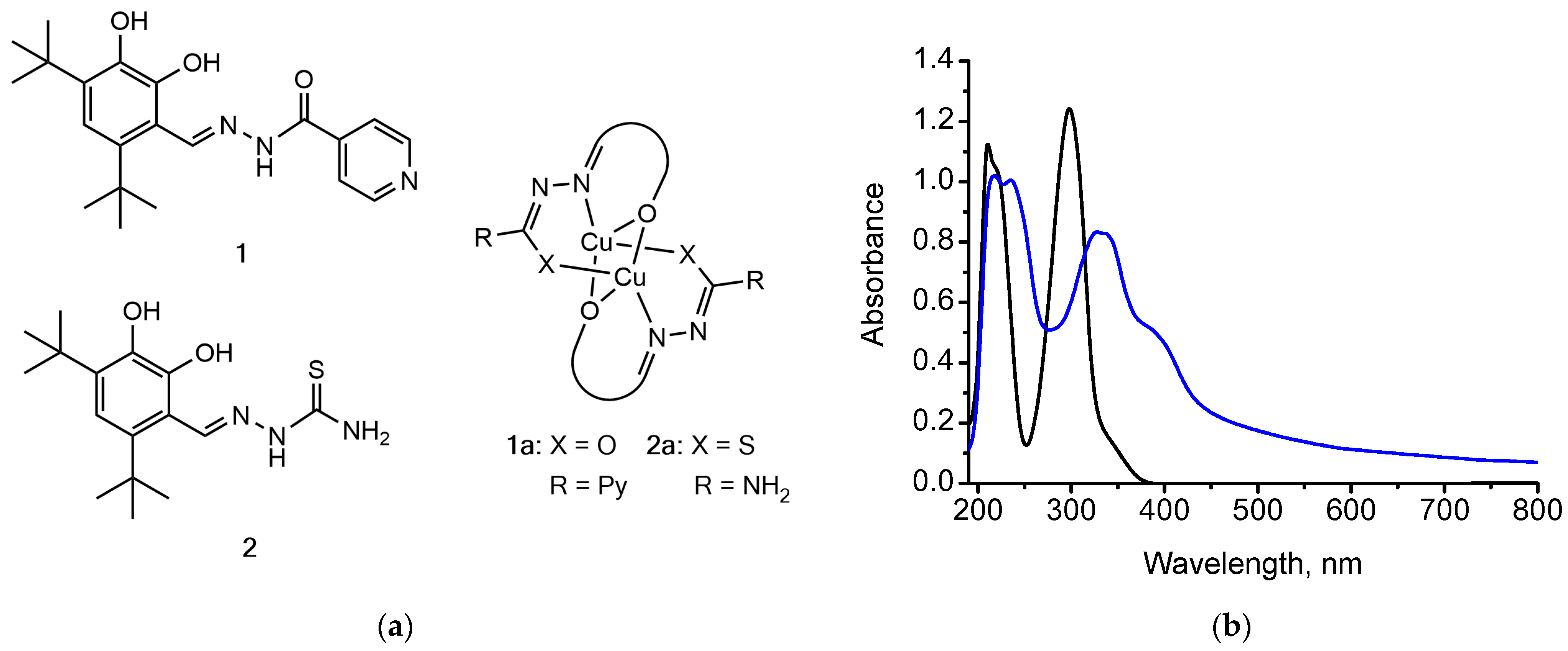
−1 appear in the spectra of the complexes, which could be assigned to valent vibrations of the formed Cu–O, Cu–N and Cu–S bonds, respectively. In the UV-Vis spectra of the ligands
1,
2, the absorption maxima assigned to azomethine group
–
* and
n–
* transitions, as well as carbonyl and thiocarbonyl groups’
n–
* transitions at 221–328 nm undergo a batochromic shift to the region of 243–384 nm upon copper(II) complexation.
ESR spectra of the copper(II) complexes
1a,
2a with the characteristic g-tensor anisotropy (
>
> 2.0023) indicate that they represent low-spin complexes with a distorted square planar geometry of the coordination core. Therefore, the complexes
1a,
2a possess binuclear coordination polyhedra [Cu
2L
2] formed by the
N,
O,
O- and
N,
O,
S-donor ligands
1 and
2, respectively (
Figure 1), which is also confirmed by the results of elemental and thermogravimetric analyses: it has been reported that
ortho-hydroxy hydrazones and thiosemicarbazones are able to coordinate copper(II) ions via hydroxy-, azomethine, and carbonyl (thiocarbonyl) groups forming binuclear systems [
21]. The formation of [Cu
2L
2] coordination polyhedron is additionally supported by the results of mass spectrometry, namely the [CuL]
+ ion peaks present in the ESI-MS (+) spectra of the complexes
1a,
2a. According to the results of XRD analysis and molar conductivity measurements, the complexes represent amorphous compounds that are essentially non-electrolytes [
22].
Antibacterial activity of the synthesized and characterized compounds
1,
1a,
2,
2a against Gram-positive (
B. subtilis,
S. saprophyticus) and Gram-negative (
P. putida,
E. coli) bacterial strains is presented in
Table 1. The obtained MIC values indicate that the copper(II) complexes of both compound
1 and
2 exhibit a more prominent antibacterial activity compared to the parent ligands. These results are in accordance with the fact that antibacterial activity is primarily necessitated by the cell membrane permeability and hence by the membranotropic properties of the compounds, which correlate with their lipophilicity [
12]. Therefore, substantially less polar and more lipophilic copper(II) complexes are more prospective in terms of antibacterial properties compared to the semi- and thiosemicarbazone
1 and
2, respectively. It should be noted that the studied compounds have displayed a considerably lower biocidal activity against Gram-negative bacterial strains compared to their Gram-positive counterparts, which could be associated with the complications arising upon penetration of antibacterial agents through the outer membrane of Gram-negative bacteria, rendering the latter more resistant [
23].
Antifungal activity of the synthesized and characterized compounds
1,
1a,
2,
2a at the concentrations of 50 and 100 μg/mL is presented in
Table 2 and
Table 3, respectively. Judging from the obtained experimental data, it may be concluded that the hydrazone and thiosemicarbazone ligands display a comparable level of antifungal activity, which is remarkably higher compared to that of their respective copper(II) complexes. This contradicts the frequently reported enhancement of antifungal activity of organic ligands upon copper(II) complexation. This observation may be attributed to the fact that copper is an essential fungal micronutrient: for instance, research on the effects of copper(II) and fluconazole on
Candida albicans has revealed that copper supplementation can paradoxically diminish the growth-inhibitory effects of fluconazole [
24]. This suggests that the parent ligands with a prominent antifungal activity could create a “window of vulnerability” for
Phytophtora that struggles to restore copper homeostasis, where additional supplementation with copper(II) could trigger compensatory responses in the fungus that ultimately reduces the overall inhibitory effect compared to the ligands alone. It should also be noted that the reason for the relatively low antifungal activity of copper(II) complexes may be associated with the reduced bioavailability of the hydrazone and thiosemicarbazone ligands upon complexation, which leads to the reduction in their initial antifungal effect. Recent evidence also suggests that copper(II) ions may induce defense responses in plants: by activating the ethylene biosynthesis pathway, copper(II) compounds may inhibit the biosynthesis of abscisic acid that negatively regulates potato immunity to
Phytophthora infestans [
25]. This nuanced copper(II)-ligand interaction underscores that in case of antifungal activity, the outcome of copper(II) complexation with an organic ligand is unpredictable: in the present case, copper(II) complexation does not yield a superior antifungal agent. With the aim of gaining a more profound insight into the possible mechanism of antifungal action of the studied compounds, their effect on the plasmid DNA has been studied by gel electrophoresis [
26]. Incubation of the plasmid DNA with the studied compounds for 1 h at 37 °C led to an increase in the relaxed DNA fraction upon formation of single-strand breaks, which suggests that nuclease activity of the compounds may contribute to their antifungal effects.