Transition Metal-Doped Cobalt Oxyhydroxide Catalysts with Enhanced Peroxymonosulfate Activation for Dye Decolorization †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of M-CoOOH
2.2.2. Decolorization of Rhodamine B
3. Results and Discussion
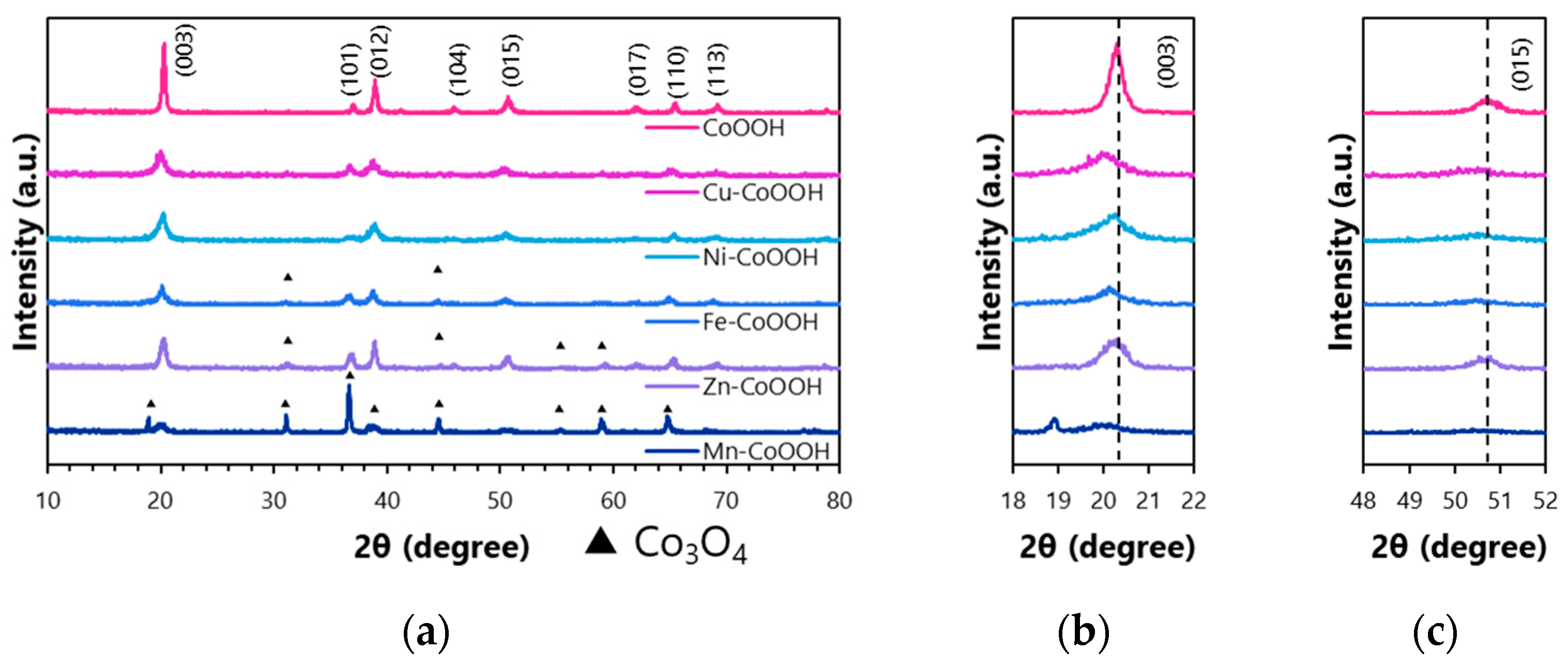
3.1. Characterization
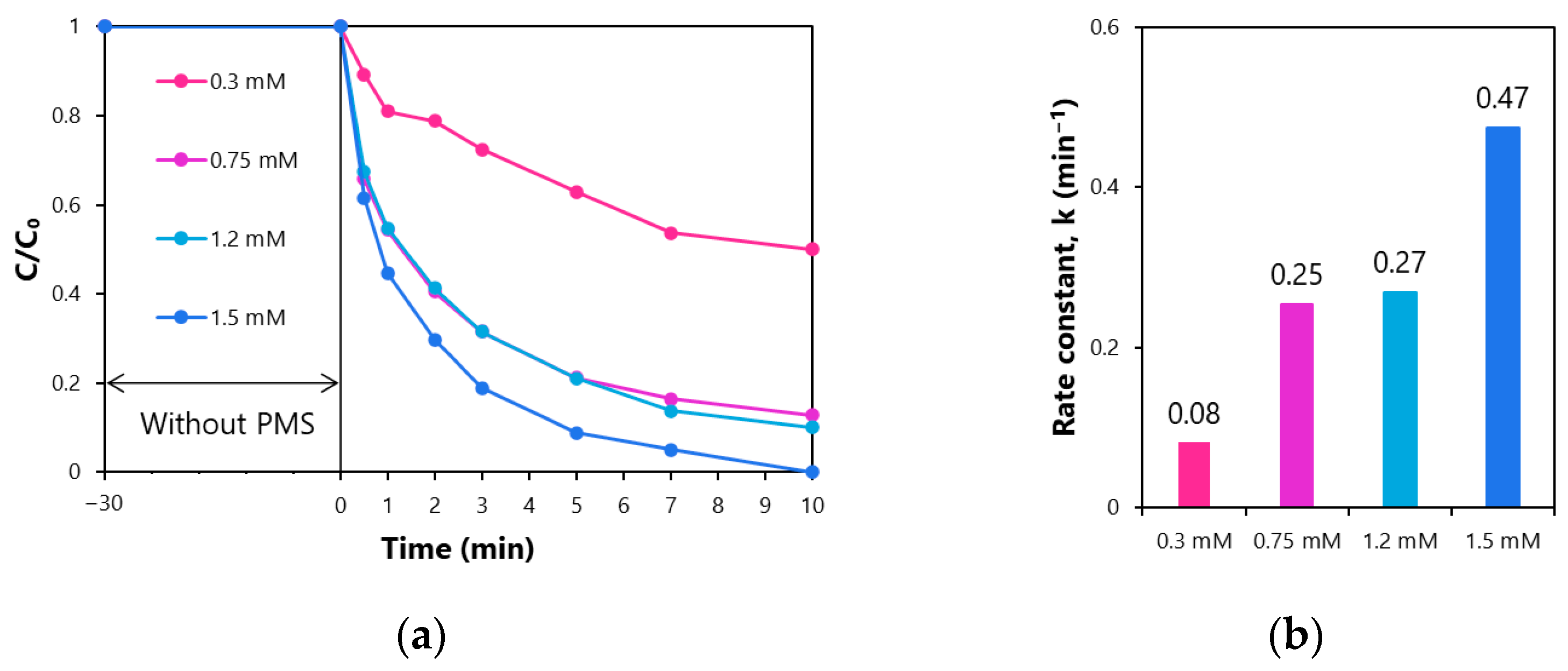
3.2. Performance of Rhodamine B Decolorization
3.3. Reusability
3.4. Decolorization of Other Dyes
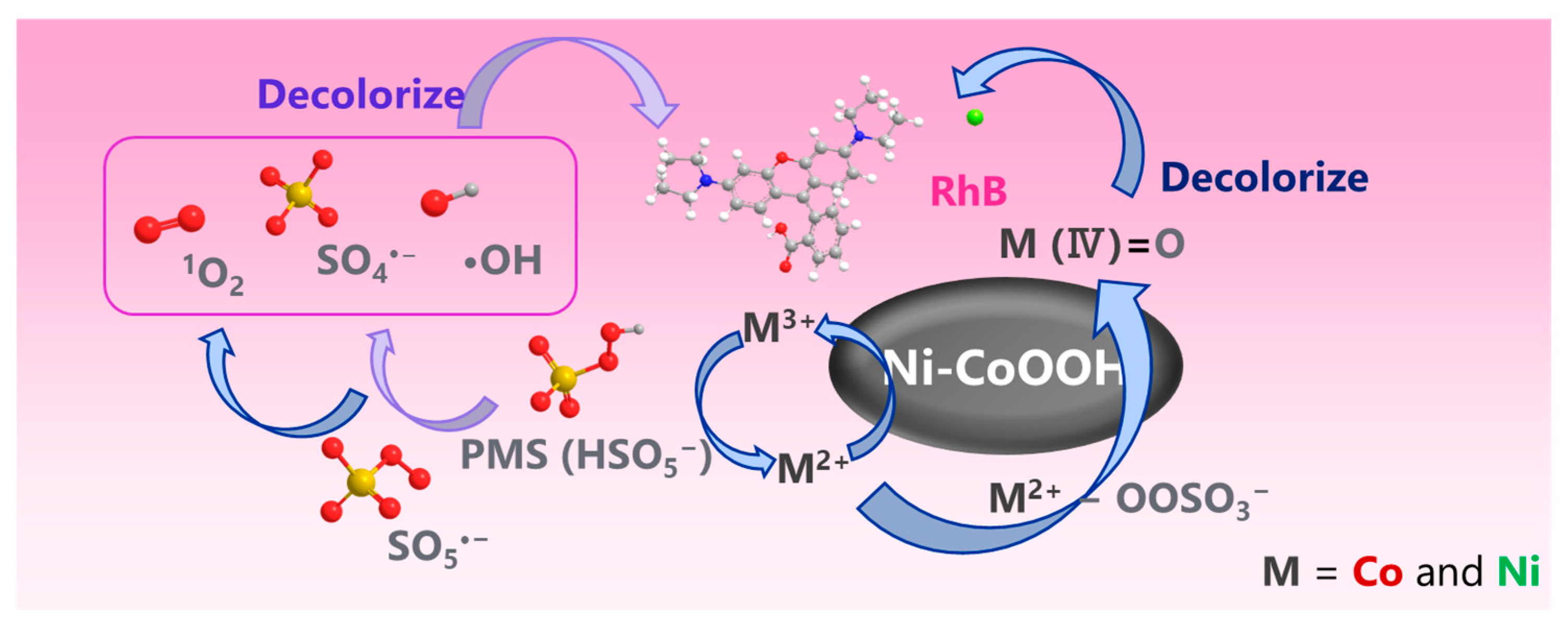
3.5. Mechanism of M-CoOOH-Activated PMS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate Radical and Its Application in Decontamination Technologies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1756–1800. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of Sulfate and Hydroxyl Radical Based Advanced Oxidation of Phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W.; Sun, W.; Cai, M.; Ye, Z.; Yang, C.; Lu, Z.; Li, C. Medium Pressure UV-Activated Peroxymonosulfate for Ciprofloxacin Degradation: Kinetics, Mechanism, and Genotoxicity. Chem. Eng. J. 2018, 345, 87–97. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and Performance of Singlet Oxygen Dominated Peroxymonosulfate Activation on CoOOH Nanoparticles for 2,4-Dichlorophenol Degradation in Water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhu, H.; Deng, J.; Shi, Z.; Zhang, H.; Li, X.; Deng, L. New Insight into Peroxymonosulfate Activation by CoAl-LDH Derived CoOOH: Oxygen Vacancies Rather than Co Species Redox Pairs Induced Process. Chem. Eng. J. 2022, 442, 136251. [Google Scholar] [CrossRef]
- Xi, T.; Li, X.; Zhang, Q.; Liu, N.; Niu, S.; Dong, Z.; Lyu, C. Enhanced Catalytic Oxidation of 2,4-Dichlorophenol via Singlet Oxygen Dominated Peroxymonosulfate Activation on CoOOH@Bi2O3 Composite. Front. Environ. Sci. Eng. 2021, 15, 55. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhan, X.; Hong, B.; Wang, X.; Tang, P.; Ding, Y.; Xia, Y.; Zeng, Y. Edge Interface Microenvironment Regulation of CoOOH/Commercial Activated Carbon Nano-Hybrids Enabling PMS Activation for Degrading Ciprofloxacin. J. Colloid. Interface Sci. 2024, 663, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, C.; Jiang, X.; Jin, X.; Peng, Y.; Kou, B.; Ni, G. Enhanced Peroxymonosulfate Activation by MnOOH/CoOOH Composites for Efficient Phenol Degradation: Mechanistic Insights and Practical Implications. J. Alloys Compd. 2025, 1010, 177678. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, C.; Zhang, W.; Liu, Z.; Li, Z.; Han, F.; Zhang, M.; Xu, F.; Zhou, W. Cu-Doped CoOOH Activates Peroxymonosulfate to Generate High-Valent Cobalt-Oxo Species to Degrade Organic Pollutants in Saline Environments. Appl. Catal. B 2024, 340, 123224. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Awad, R. Study of the Structural and Physical Properties of Co3O4 Nanoparticles Synthesized by Co-Precipitation Method. J. Supercond. Nov. Magn. 2020, 33, 1395–1404. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous Activation of Peroxymonosulfate by Fe-Co Layered Doubled Hydroxide for Efficient Catalytic Degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.H. A-MnO2/Palygorskite Composite as an Effective Cat-alyst for Heterogeneous Activation of Peroxymonosulfate (PMS) for the Degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Chen, D.; Yuvaraja, G.; Mehmood, S. Facilely Synthesized Cobalt Doped Hydroxyapatite as Hydroxyl Promoted Peroxymonosulfate Activator for Degradation of Rhodamine B. J. Hazard. Mater. 2020, 384, 121447. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Long, Y.; Zhao, S.; Wang, P.; Xie, F.; Huang, J.; Han, B.; Zhang, Z.; Zhang, B.P. Reduced Fe, Mn-Based Catalyst with Dual Reaction Sites for Rapid Decolorization Treatment via Fenton-like Reactions. Appl. Surf. Sci. 2023, 616, 156522. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W.; Cheng, X.; Liu, H. Activation of Peroxymonosulfate by BiVO4 under Visible Light for Degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.; Sun, T.; Cai, C.; Dong, Y. The Preparation of MoS2/δ-FeOOH and Degradation of RhB under Visible Light. J. Environ. Chem. Eng. 2023, 11, 110353. [Google Scholar] [CrossRef]


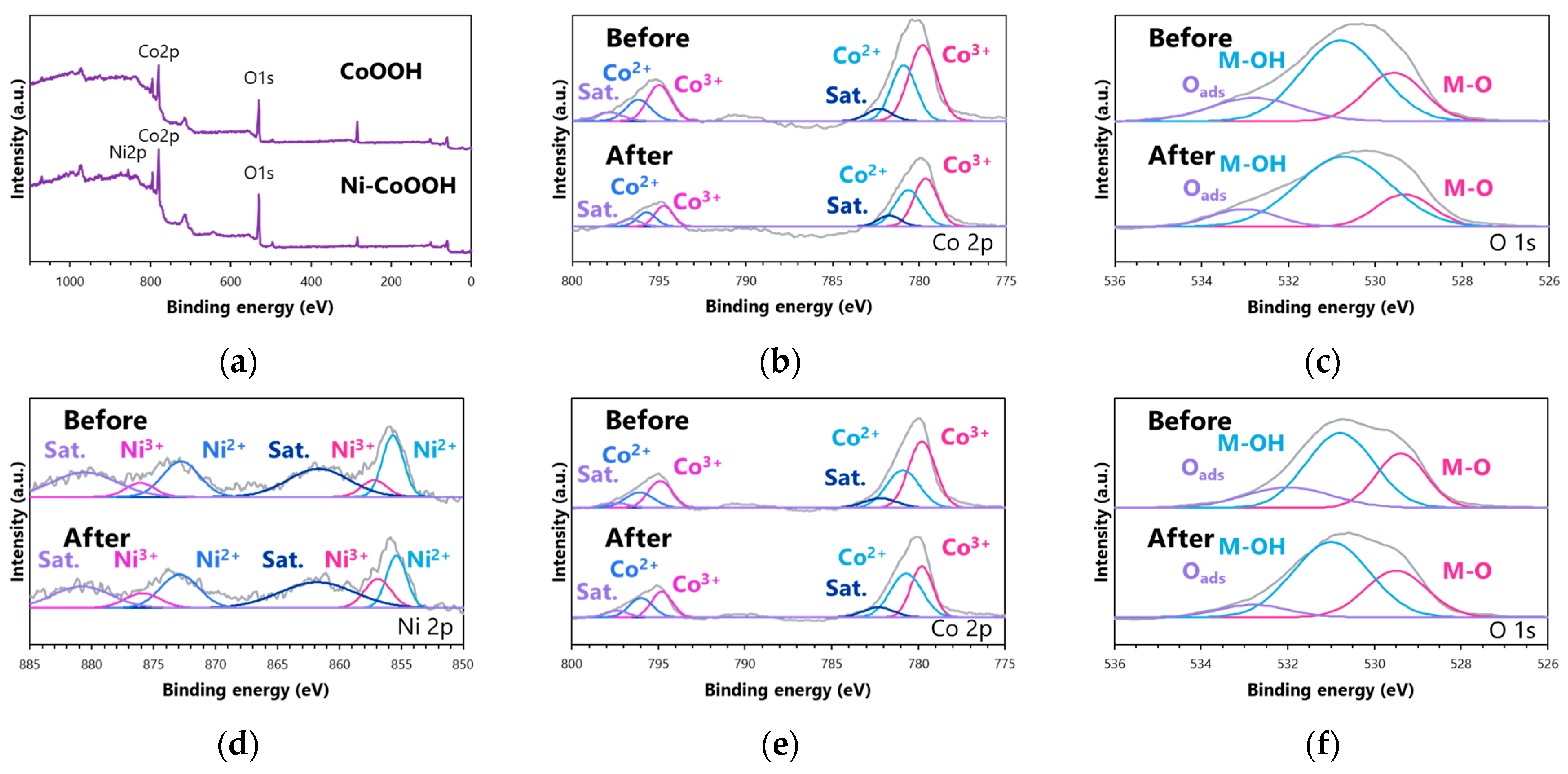
| CoOOH | Ni-CoOOH | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co3+ | Co2+ | Oads | M-OH | M-O | Co3+ | Co2+ | Ni3+ | Ni2+ | Oads | M-OH | M-O | |
| Before (%) | 60.5 | 39.5 | 18.1 | 56.7 | 25.2 | 59.3 | 40.7 | 23.9 | 76.1 | 18.7 | 52.8 | 28.5 |
| After (%) | 54.6 | 45.4 | 11.8 | 68.9 | 19.2 | 47.4 | 52.6 | 35.4 | 64.6 | 9.1 | 60.4 | 30.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, R.; Katsumata, H.; Tateishi, I.; Furukawa, M.; Kaneco, S. Transition Metal-Doped Cobalt Oxyhydroxide Catalysts with Enhanced Peroxymonosulfate Activation for Dye Decolorization. Chem. Proc. 2025, 17, 8. https://doi.org/10.3390/chemproc2025017008
Yamamoto R, Katsumata H, Tateishi I, Furukawa M, Kaneco S. Transition Metal-Doped Cobalt Oxyhydroxide Catalysts with Enhanced Peroxymonosulfate Activation for Dye Decolorization. Chemistry Proceedings. 2025; 17(1):8. https://doi.org/10.3390/chemproc2025017008
Chicago/Turabian StyleYamamoto, Rina, Hideyuki Katsumata, Ikki Tateishi, Mai Furukawa, and Satoshi Kaneco. 2025. "Transition Metal-Doped Cobalt Oxyhydroxide Catalysts with Enhanced Peroxymonosulfate Activation for Dye Decolorization" Chemistry Proceedings 17, no. 1: 8. https://doi.org/10.3390/chemproc2025017008
APA StyleYamamoto, R., Katsumata, H., Tateishi, I., Furukawa, M., & Kaneco, S. (2025). Transition Metal-Doped Cobalt Oxyhydroxide Catalysts with Enhanced Peroxymonosulfate Activation for Dye Decolorization. Chemistry Proceedings, 17(1), 8. https://doi.org/10.3390/chemproc2025017008






