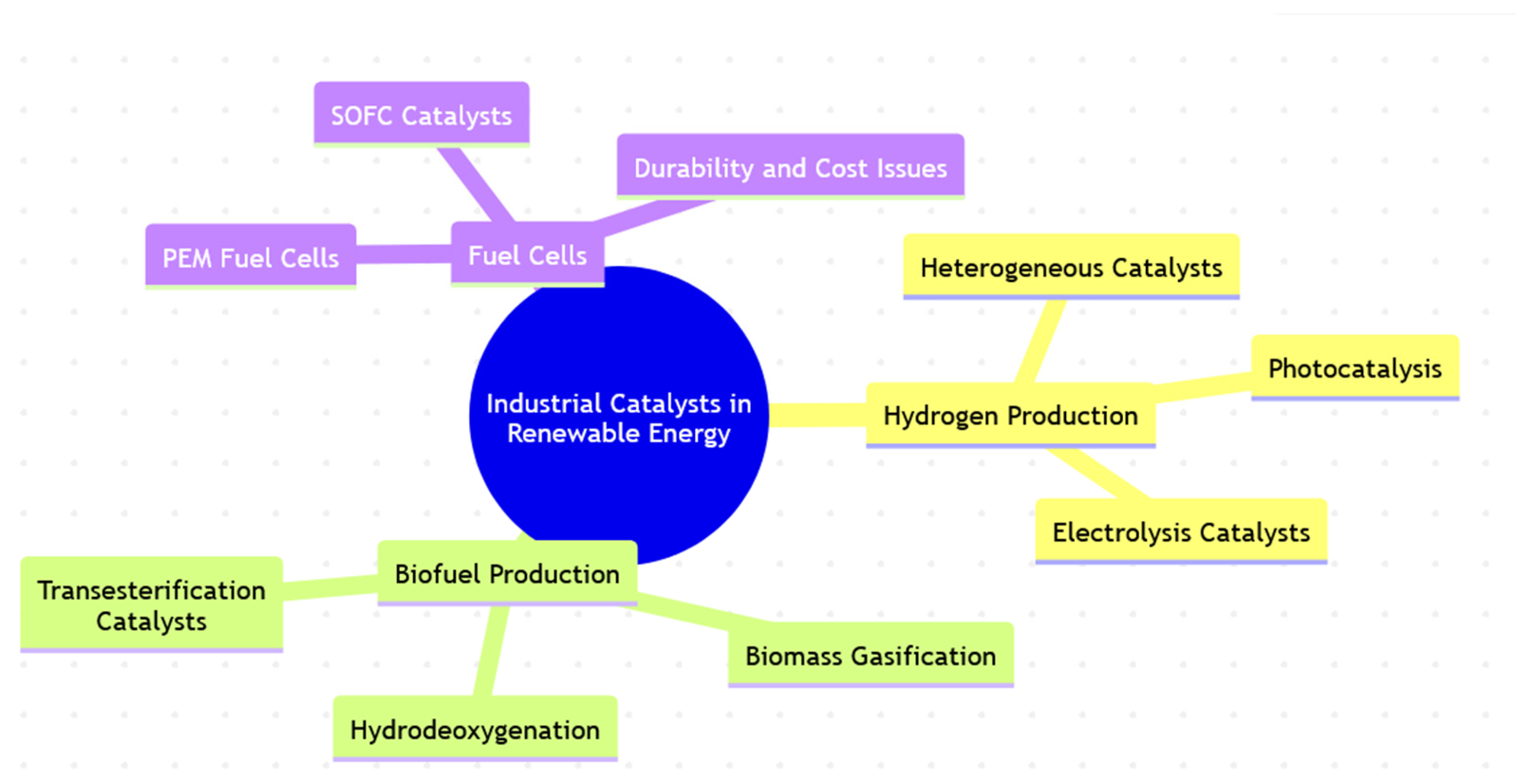
The Role of Industrial Catalysts in Accelerating the Renewable Energy Transition †
Abstract
1. Introduction
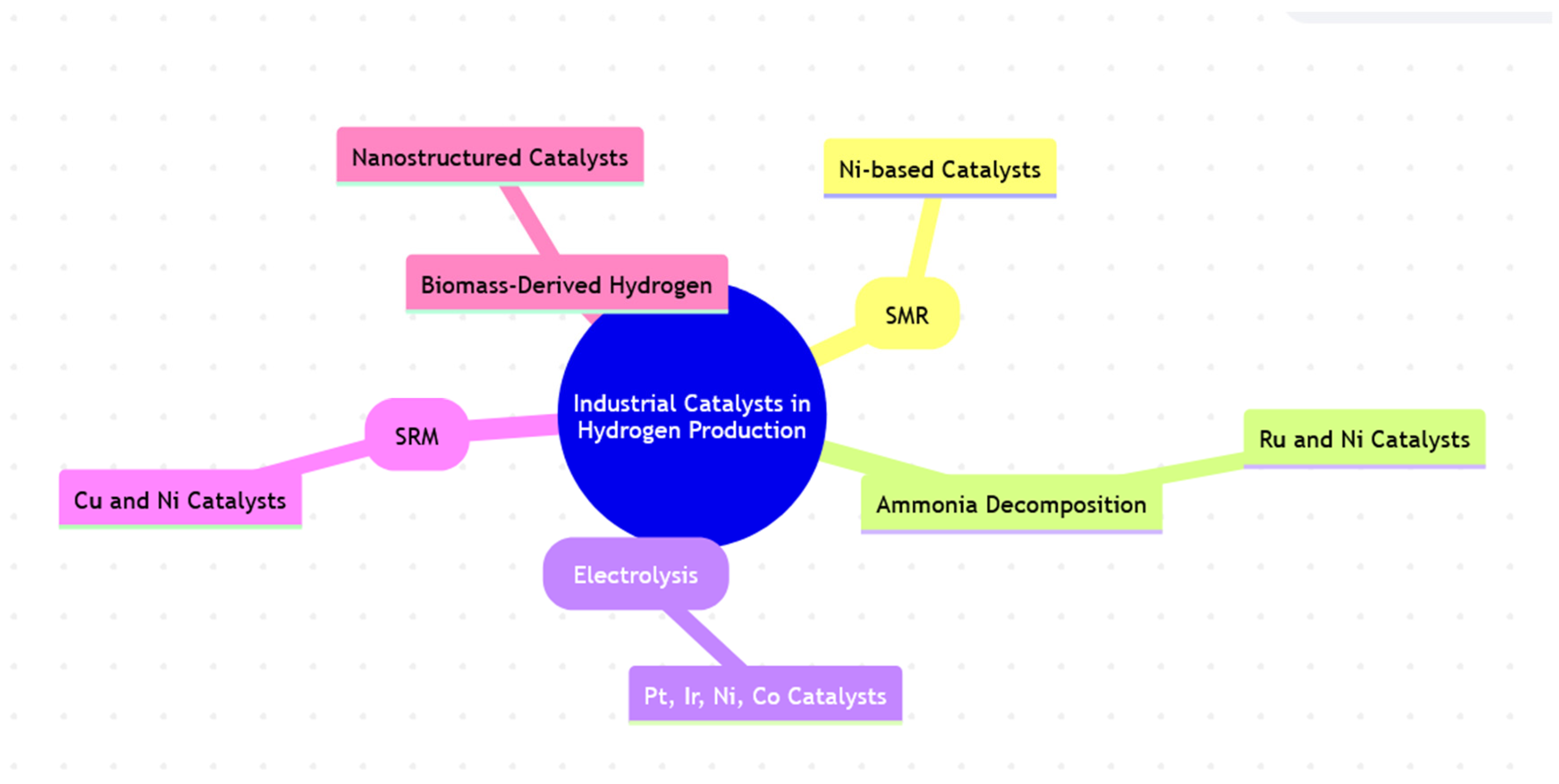
2. Industrial Catalysts in Hydrogen Production
2.1. Steam Methane Reforming (SMR)
- -
- -
- Advancements: To address these challenges, bimetallic catalysts—comprising Ni and metals such as Co, Cu, or noble elements—have demonstrated enhanced thermal stability, coke resistance, and catalytic activity. These advances contribute to higher hydrogen yield and longer catalyst life [12].
2.2. Ammonia Decomposition
- -
- Catalysts: Effective ammonia decomposition requires catalysts with a balance of active components (like Ru or Ni), robust supports, and promoters to boost performance. Optimized reactor designs, such as membrane or packed-bed reactors, are employed for improved conversion [13].
- -
- Future Trends: Research is moving toward low-cost and scalable catalysts with greater selectivity and stability under practical operating conditions [14].
2.3. Water Splitting (Electrolysis)
- -
- Catalysts: Platinum group metals (PGMs) like Pt and Ir offer excellent efficiency but are costly and scarce. As alternatives, non-precious metal catalysts—including Ni, Fe, Co, and their oxides or phosphides—are being developed for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) [15].
- -
- Innovations: Trimetallic catalysts, combining three active metals, have demonstrated superior catalytic synergy, stability, and long-term durability, making them suitable for scalable electrolyzers [16].
2.4. Methanol Steam Reforming (SRM)
- -
- Catalysts: Cu and Ni-based catalysts are widely used for SRM due to their good hydrogen yield at moderate temperatures. However, challenges like coke formation and metal agglomeration impact their effectiveness [17].
- -
- Solutions: Novel catalyst supports such as carbon nanotubes and metal–organic frameworks (MOFs), as well as bimetallic configurations, are being explored to improve dispersion, reduce deactivation, and boost performance [17].
2.5. Biomass-Derived Hydrogen Production
- -
- Catalysts: Nanostructured and multicomponent catalysts are being developed to handle complex feedstocks like bio-oil and glycerol. These catalysts enhance selectivity, resist poisoning, and improve reforming efficiency [16].
- -
- Applications: Such catalysts are highly versatile and can be applied in both aqueous-phase reforming and photo-electrochemical water splitting, making them essential for decentralized, renewable hydrogen systems.
3. Industrial Catalysts in Biofuel Production
3.1. Types of Catalysts in Biofuel Production
3.1.1. Homogeneous Catalysts
- -
- -
3.1.2. Heterogeneous Catalysts
- -
- -
3.1.3. Biocatalysts
- -
3.2. Roles and Advantages of Catalysts in Biofuel Production
4. Industrial Catalysts in Fuel Cells: Efficiency Improvements
5. Challenges and Future Outlook of Industrial Catalysts in the Energy Sector
6. Ecological Footprint of Different Types of Catalysts
7. Results and Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durairaj, S.; Annamalai, P.; Dhanalakshmi, R.; Ghosh, D. Advances and outlook of Ti3C2-based catalysts for electrocatalytic hydrogen production: A comprehensive overview. Energy Fuels 2024, 38, 20258–20284. [Google Scholar] [CrossRef]
- Swami, S.M.; Abraham, M. An integrated catalytic process for conversion of biomass to hydrogen. In Proceedings of the AIChE Annual Meeting, Cincinnati, OH, USA, 30 October–4 November 2005; p. 12061. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-33645647644&partnerID=40 (accessed on 6 February 2025).
- D’Ambrosio, V.; La Parola, V.; Francesca Liotta, L.; Roberto, E.; Carraro, G.; Savio, L.; Comparelli, R.; Lucia Curri, M.; Pastore, C. Catalytic hydrogenation of waste-derived lipids: A route to producing sustainable drop-in biofuels by using Re/TiO2 catalysts. Chem. Eng. J. 2024, 499, 156648. [Google Scholar] [CrossRef]
- Berchthold, C.; Geisbauer, A. Will power and waste-to-X enable the worldwide energy transition to renewables? Monatshefte Chem. 2024, 155. [Google Scholar] [CrossRef]
- Smith, W.A.; Burdyny, T.; Vermaas, D.A.; Geerlings, H. Pathways to industrial-scale fuel out of thin air from CO2 electrolysis. Joule 2019, 3, 1822–1834. [Google Scholar] [CrossRef]
- Dymerska, A.; Kukułka, W.; Biegun, M.; Mijowska, E. Spinel of nickel-cobalt oxide with rod-like architecture as electrocatalyst for oxygen evolution reaction. Materials 2020, 13, 3918. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.T.S.T.; Lopes, O.F.; Dias, E.H.; Torres, J.A.; Nogueira, A.E.; Faustino, L.A.; Prado, F.S.; Patrocínio, A.O.T.; Ribeiro, C. CO2 reduction to hydrocarbons and oxygenates: Fundamentals, strategies and challenges. Química Nova 2021, 44, 963–981. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, D.; Lin, C.-Y.; Zhu, Y.; Shen, Y.; Zhang, J.; Han, X.; Zhang, L.; Xia, Z. Catalytic mechanisms and design principles for single-atom catalysts in highly efficient CO2 conversion. Adv. Energy Mater. 2019, 9, 1902625. [Google Scholar] [CrossRef]
- Brandao, D.S.; de Souza, F.G.; Maranhão, F.D.S., Jr.; Pal, K.; de Paula Pereira, M.C.; Torres, A.C.; Silva, G.B.; Peçanha, T.D.N.; e Silva, S.E.C.; Carelo, J.C.; et al. Biodiesel synthesis using magnetizable geopolymer as heterogeneous catalysts nanocomposite assisted by artificial intelligence. Top. Catal. 2024, 67, 785–809. [Google Scholar] [CrossRef]
- Du, X.; Zhang, R.; Li, D.; Hu, C.; Garcia, H. Molybdenum carbide as catalyst in biomass derivatives conversion. J. Energy Chem. 2022, 73, 68–87. [Google Scholar] [CrossRef]
- Zolghadri, S.; Kiani, M.R.; Kamandi, R.; Rahimpour, M.R. Enhanced hydrogen production in steam methane reforming: Comparative analysis of industrial catalysts and process optimization. J. Energy Inst. 2024, 113, 101541. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Kotob, E.; Abdulhakam, A.; Taialla, O.A.; Awad, M.M.; Hussain, I.; Alhooshani, K.R.; Ganiyu, S.A. Recent advances in bimetallic catalysts for methane steam reforming in hydrogen production: Current trends, challenges, and future prospects. Chem. Asian J. 2024, 19, e202300641. [Google Scholar] [CrossRef]
- Chen, R.; Wang, R.; Lu, X.; Zhao, S.; Liao, Y.; Pan, H.; Zhan, Z.; Tang, H. NH3 to H2, exploration from pyrolytic key materials to device structure design. J. Ind. Eng. Chem. 2024, 134, 1–16. [Google Scholar] [CrossRef]
- Wang, T.; Xie, H.; Chen, M.; D’Aloia, A.; Cho, J.; Wu, G.; Li, Q. Precious metal-free approach to hydrogen electrocatalysis for energy conversion: From mechanism understanding to catalyst design. Nano Energy 2017, 42, 69–89. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Arandiyan, H.; Song, G.; Sun, H.; Sabri, Y.; Zhao, C.; Shao, Z.; Kawi, S. Advancing catalysts by stacking fault defects for enhanced hydrogen production: A review. Adv. Mater. 2024, 36, 2313378. [Google Scholar] [CrossRef] [PubMed]
- Fajín, J.L.C.; Cordeiro, M.N.D.S. Renewable hydrogen production from biomass derivatives or water on trimetallic based catalysts. Renew. Sustain. Energy Rev. 2024, 189, 113909. [Google Scholar] [CrossRef]
- Rostami, M.; Farajollahi, A.H.; Amirkhani, R.; Farshchi, M.E. A review study on methanol steam reforming catalysts: Evaluation of the catalytic performance, characterizations, and operational parameters. AIP Adv. 2023, 13, 030701. [Google Scholar] [CrossRef]
- He, Z.-H.; Jiang, C.-S.; Wang, Z.-Y.; Wang, K.; Sun, Y.-C.; Yao, M.-Q.; Li, Z.-H.; Liu, Z.-T. Catalytic hydrodeoxygenation of biomass-derived oxygenates to bio-fuels over Co-based bimetallic catalysts. Sustain. Energy Fuels 2020, 4, 4558–4569. [Google Scholar] [CrossRef]
- Nganda, A.; Srivastava, P.; Lamba, B.Y.; Pandey, A.; Kumar, M. Advances in the fabrication, modification, and performance of biochar, red mud, calcium oxide, and bentonite catalysts in waste-to-fuel conversion. Environ. Res. 2023, 232, 116284. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Le, H.S.; Le, H.C.; Le, D.T.N.; Cao, D.N.; Truong, T.H.; Tran, V.D. Metal-organic frameworks as potential catalysts for biodiesel production and biomass conversion: Mechanism and characteristics. Ind. Crops Prod. 2024, 211, 118232. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Application of catalysts in biodiesel production. In Biodiesel Technology and Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 85–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Deng, T.; Cheng, J.; Ma, P. Production of biodiesel with metal-oxide-based catalysts. In Advanced Catalysis for Drop-in Chemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 155–191. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Zik, N.A.F.A.; Sulaiman, S.; Jamal, P. Thermally produced nano catalyst for biodiesel production: A review. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 52, 139–147. [Google Scholar]
- Mofijur, M.; Siddiki, S.Y.A.; Shuvho, M.B.A.; Djavanroodi, F.; Fattah, I.M.R.; Ong, H.C.; Chowdhury, M.A.; Mahlia, T.M.I. Effect of nanocatalysts on the transesterification reaction of first, second and third generation biodiesel sources—A mini-review. Chemosphere 2021, 270, 128642. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.-Y. Biodiesel process uses nanoparticle catalyst. Ind. Bioprocess. 2006, 28, 2. [Google Scholar]
- Kalita, P.; Basumatary, B.; Saikia, P.; Das, B.; Basumatary, S. Biodiesel as renewable biofuel produced via enzyme-based catalyzed transesterification. Energy Nexus 2022, 6, 100087. [Google Scholar] [CrossRef]
- Cabello, C.; Rincon, S.; Zeped, A. Types of heterogeneous catalysts used for biodiesel production [Catalizadores heterogéneos utilizados para la obtención de biodiesel]. Afinidad 2017, 74, 51–59. [Google Scholar]
- Hussain, A.; Ghaffar, I.; Sattar, S.; Muneeb, M.; Hasan, A.; Deepanraj, B. Eco-friendly catalysts revolutionizing energy and environmental applications: An overview. Top. Catal. 2025, 68, 487–509. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Qin, B.; Zhou, Z.; Zhou, S.; Li, K.; Wei, Z. Mechanistic studies toward the rational design of oxide catalysts for carbon dioxide hydrogenation. In Annual Reports in Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 17, pp. 211–270. [Google Scholar] [CrossRef]
- Parkash, A.; Solangi, N.; Seehar, T.H.; Zhang, G.; Akram, M.; Ali, S. Review—Heteroatom-doped high porous carbon metal free nanomaterials for energy storage and conversion. ECS J. Solid State Sci. Technol. 2022, 11, 091006. [Google Scholar] [CrossRef]
- Ren, X.; Liu, B.; Liang, X.; Wang, Y.; Lv, Q.; Liu, A. Review—Current progress of non-precious metal for ORR based electrocatalysts used for fuel cells. J. Electrochem. Soc. 2021, 168, 044521. [Google Scholar] [CrossRef]
- Sharma, R.; Cherusseri, J.; Kar, K.K. Polymer electrolyte membrane fuel cells: Role of carbon nanotubes/graphene in cathode catalysis. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 361–390. [Google Scholar] [CrossRef]
- Battersby, S. Nanotubes outshine platinum as fuel-cell catalysts. New Sci. 2009, 201, 22. [Google Scholar] [CrossRef]
- Tian, M.; Shi, S.; Shen, Y.; Yin, H. PtRu alloy nanoparticles supported on nanoporous gold as an efficient anode catalyst for direct methanol fuel cell. Electrochim. Acta 2019, 293, 390–398. [Google Scholar] [CrossRef]
- Mergel, J.; Wippermann, K. Corrosion in fuel cells [Korrosion in Brennstoffzellen]. Galvanotechnik 2009, 100, 535–540. [Google Scholar]
- Zhang, Y.; Wilkinson, D.P.; Taghipour, F. Performance analysis of an air-breathing micro-direct methanol fuel cell with an extended anode region. Fuel Cells 2020, 20, 634–642. [Google Scholar] [CrossRef]
- Chang, Z.; Guan, L.; Zhang, J.; Zhang, W.; Ma, Q.; Shah, A.; Xing, L.; Su, H.; Xu, Q. Construction of gradient catalyst layer anode by incorporating covalent organic framework to improve performance of direct methanol fuel cells. Int. J. Hydrogen Energy 2022, 47, 37013–37024. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.; Baik, C.; Cho, Y.-H.; Pak, C. High performance carbon-supported IrRu alloy catalyst for the in an alkaline anion-exchange membrane fuel cell. J. Alloys Compd. 2021, 868, 159205. [Google Scholar] [CrossRef]
- Heo, P.; Nagao, M.; Sano, M.; Hibino, T. A high performance Pt-free anode for intermediate-temperature fuel cells. ECS Trans. 2006, 3, 453–458. [Google Scholar] [CrossRef]
- Spivey, J.J.; Roberts, G.W.; Lamb, H.H.; Chin, P.; Sigmon, S.; Silletti, B.; Wang, X.; Sun, X.; Goodwin, J.G., Jr.; Butcher, K. Catalytic approaches to the development of clean energy technologies. Am. Chem. Soc. Div. Pet. Chem. 2004, 49, 341–342. [Google Scholar]
- Kolle-Görgen, E.; Fortunato, G.; Ledendecker, M. Catalyst stability in aqueous electrochemistry. Chem. Mater. 2022, 34, 10223–10236. [Google Scholar] [CrossRef]
- Clayton, J.; Jokil, N.H.; Topsøe, H. Powder testing boosts catalyst making. Chem. Process. 2021, 83, 9–15. [Google Scholar]
- Fechete, I.; Wang, Y.; Védrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Kapusta, S.; Balzano, L.; Te Riele, P. Nanotechnology applications in oil and gas exploration and production. In Proceedings of the International Petroleum Technology Conference 2011 (IPTC 2011), Bangkok, Thailand, 15–17 November 2011; Available online: https://www.scopus.com/pages/publications/85053774129 (accessed on 6 February 2025).
- Kapusta, S.; Balzano, L.; Te Riele, P. Nanotechnology applications in oil and gas exploration and production. In Proceedings of the Society of Petroleum Engineers—International Petroleum Technology Conference 2012 (IPTC 2012), Bangkok, Thailand, 7–9 February 2012; Volume 3, pp. 2770–2774. Available online: https://www.scopus.com/pages/publications/84861416510 (accessed on 6 February 2025).
- Rostrup-Nielsen, J.R. Challenges to industrial catalysis. Catal. Today 2023, 423, 113878. [Google Scholar] [CrossRef]
- Hocking, R.K.; Chang, S.L.Y.; MacFarlane, D.R.; Spiccia, L. Preparation and characterization of catalysts for clean energy: A challenge for X-rays and electrons. Aust. J. Chem. 2012, 65, 608–614. [Google Scholar] [CrossRef]
- Centi, G.; Lanzafame, P.; Perathoner, S. Introduction and general overview. In Catalysis for Alternative Energy Generation; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J.-D. Future challenges in heterogeneous catalysis: Understanding catalysts under dynamic reaction conditions. ChemCatChem 2017, 9, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.M.; Sangwai, J.S. Interaction of heavy crude oil and nanoparticles for heavy oil upgrading. In Green Energy and Technology; Springer: Cham, Switzerland, 2020; pp. 231–255. [Google Scholar] [CrossRef]
- Rüzgar, D.; Yıldız, D.; Kaya, Ş.; Martinez, J.V.; Yeşilbursa, G.; Demir Kıvrak, H. Waste ceramic frit as novel catalyst triggering sodium borohydride methanolysis reaction. Int. J. Hydrogen Energy 2024, 56, 1038–1048. [Google Scholar] [CrossRef]
- Rey, I.; Barrio, V.L.; Agirre, I. Environmental assessment of a hydrogen supply chain using LOHC system with novel low-PGM catalysts: A life cycle approach. Int. J. Hydrogen Energy 2025, 142, 616–626. [Google Scholar] [CrossRef]
- Christopher Selvam, D.; Devarajan, Y.; Raja, T.; Natrayan, L. Green hydrogen from waste: Exploring the promise of sustainable catalysis. BioNanoScience 2025, 15, 433. [Google Scholar] [CrossRef]
- Arfelis, S.; Malpartida, I.; Lair, V.; Caldeira, V.; Sazdovski, I.; Bala, A.; Fullana-i-Palmer, P. Life cycle assessment on calcium zincate production methods for rechargeable batteries. Sci. Total Environ. 2023, 866, 161094. [Google Scholar] [CrossRef]
- Medina, O.E.; Amell, A.A.; López, D.; Santamaría, A. Comprehensive review of nickel-based catalysts advancements for CO2 methanation. Renew. Sustain. Energy Rev. 2025, 207, 114926. [Google Scholar] [CrossRef]
- Frazier, R.S.; Jin, E.; Kumar, A. Life cycle assessment of biochar versus metal catalysts used in syngas cleaning. Energies 2015, 8, 621–644. [Google Scholar] [CrossRef]
- Damian, C.S.; Devarajan, Y.; Ravikumar, J.; Raja, T. Nanocatalysts in biodiesel production: Advancements, challenges, and sustainable solutions. ChemBioEng Rev. 2025, 12, e202400055. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Putri, N.A.; Farahita, A.M.; Manullang, H.B.; Christa Jaya, D.E.; Amenaghawon, A.N.; Darmokoesoemo, H.; Mahfud, M. Sustainable nickel recovery: Advancing bioleaching techniques for secondary sources in renewable energy and circular economy applications. Sustain. Chem. Pharm. 2025, 46, 102094. [Google Scholar] [CrossRef]
| Catalyst Type | Examples | Advantages | Challenges |
|---|---|---|---|
| Homogeneous Acid | H2SO4, H3PO4 | High reaction rates | Difficult separation, high waste generation |
| Homogeneous Base | NaOH, KOH | Effective for high-quality feedstocks | Ineffective for low-quality feedstocks |
| Heterogeneous Acid | Sulfated zirconia, alumina | Reusability, easy separation | Lower activity compared to base catalysts |
| Heterogeneous Base | CaO, MgO, ZrO2 | High stability, reusability | Cannot esterify large amounts of FFAs |
| Biocatalysts | Lipases | High selectivity, mild conditions | High cost, limited industrial use |
| Nanocatalysts | Metal oxides, nanoparticles | High surface area, catalytic efficiency | Thermal stability issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borthakur, P.P.; Borthakur, B. The Role of Industrial Catalysts in Accelerating the Renewable Energy Transition. Chem. Proc. 2025, 17, 6. https://doi.org/10.3390/chemproc2025017006
Borthakur PP, Borthakur B. The Role of Industrial Catalysts in Accelerating the Renewable Energy Transition. Chemistry Proceedings. 2025; 17(1):6. https://doi.org/10.3390/chemproc2025017006
Chicago/Turabian StyleBorthakur, Partha Protim, and Barbie Borthakur. 2025. "The Role of Industrial Catalysts in Accelerating the Renewable Energy Transition" Chemistry Proceedings 17, no. 1: 6. https://doi.org/10.3390/chemproc2025017006
APA StyleBorthakur, P. P., & Borthakur, B. (2025). The Role of Industrial Catalysts in Accelerating the Renewable Energy Transition. Chemistry Proceedings, 17(1), 6. https://doi.org/10.3390/chemproc2025017006







