Abstract
Commercially available bismuth subcarbonate (Bi2O2CO3) was treated with nitric acid and the surfactant cetyltrimethylammonium bromide. The treated catalysts exhibited enhanced photocatalytic activity compared to pure Bi2O2CO3 in the decolorization of rhodamine B (RhB) under visible light irradiation. The absorbance at 554 nm gradually decreased over time and disappeared completely within 80 min. The crystal structure, morphology, and optical properties of the samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-Vis diffuse reflectance spectroscopy (DRS), and photoluminescence (PL) spectroscopy. The improved photocatalytic activity of the treated catalysts was attributed to partial carbonate removal and the formation of Bi5+ species. Scavenger experiments indicated that superoxide radicals (·O2−) and photogenerated holes (h+) played significant roles in the photocatalytic decolorization of RhB.
1. Introduction
Semiconductor photocatalysis is regarded as an environmentally friendly and promising approach to addressing water pollution [1]. Among different photocatalysts, Bi2O2CO3 (BOC) features a unique layered structure that can enhance the separation efficiency of photogenerated electron–hole pairs [2]. In particular, the orthogonal arrangement of [Bi2O2]2+ and [CO3]2− layers generates an internal electric field in the vertical direction, thereby promoting photocatalytic activity [3]. However, the wide optical band gap (2.8 eV–3.8 eV) of BOC limits its absorption of visible light, and the recombination rate of electron–hole pairs remains high [4]. Therefore, it is important to narrow the band gap and improve visible-light responsiveness. Previous studies have shown that appropriate chemical treatments can effectively improve the photocatalytic activity of BOC [5,6]. However, most of these studies have focused on modifications applied during the synthesis process, while relatively few have addressed post-synthesis modifications. Treatment of the prepared catalyst offers the advantage of avoiding the formation of undesired phases and preserving the original crystal structure. In this study, BOC was treated with different concentrations of nitric acid and different bromine sources, and its photocatalytic performance was evaluated through the decolorization of RhB. Among the prepared samples, the one treated with 50 mmol/L nitric acid and an equimolar amount of cetyltrimethylammonium bromide (CTAB)—a bromine source that also functions as a surfactant—(hereafter referred to as BOC-N50 CB1.0) exhibited the highest photocatalytic activity. Characterization results revealed that nitric acid acted as an oxidant agent, partially converting Bi3+ to Bi5+, and CTAB promoted partial desorption of carbonate species. These structural and chemical modifications are considered to contribute to the enhanced photocatalytic performance. Finally, the dominant reactive species involved in the RhB decolorization process by BOC-N50 CB1.0 were investigated using scavengers for ·O2−, h+, and ·OH, and a possible photocatalytic mechanism was proposed.
2. Materials and Methods
2.1. Materials
All chemicals used in this study were of analytical grade and were used without further purification. Cetyltrimethylammonium bromide (CTAB, [CH3(CH2)15N(CH3)3]Br), potassium bromide (KBr), sodium bromide (NaBr), p-benzoquinone (BQ, C6H4O2), and ammonium oxalate monohydrate (AO, (NH4)2C2O4·H2O) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Bismuth carbonate (Bi2O2CO3) and tert-butyl alcohol (TBA, (CH3)3COH) were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Rhodamine B (C28H31ClN2O3) was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Pure water was obtained from an ultrapure water production system (Advantec MFS, Inc., Tokyo, Japan).
2.2. BOC Pretreatment Method
First, we will show the pretreatment method for BOC that had the best activity. CTAB (1.0 mmol) was dissolved in 50 mL of 50 mmol/L nitric acid solution. BOC (1 mmol) was added to the solution and the mixture was stirred for 2 h. The resulting product was washed with distilled water and ethanol and dried in vacuum at 60 °C for 12 h. The prepared photocatalyst was designated BOC-N50 CB1.0. For comparison, reference samples treated with nitric acid only (BOC-N50) and CTAB only (BOC-CB0.5) were also prepared. Depending on the amount of CTAB added during synthesis, the samples were designated BOC-N50 CBx (x = 0.5, 1.0, 1.5 mmol). In addition, catalysts synthesized using NaBr (NB, 0.5 mmol) or KBr (KB, 0.5 mmol) instead of CTAB were designated BOC-N50 NB0.5 and BOC-N50 KB0.5, respectively. Furthermore, samples prepared with different concentrations of nitric acid were designated BOC-Ny CB0.5 (y = 10, 50, 100 mmol/L).
2.3. Characterizations
Absorbance spectra were measured using a UV-Visible spectrophotometer (V-750, JASCO Co., Tokyo, Japan). The crystal structures of the prepared samples were characterized by X-ray diffraction (XRD) over a 2θ range of 10–80°, using an Ultima IV diffractometer (Rigaku Co., Tokyo, Japan) with Cu Kα radiation (λ = 0.15406 nm). The valence states of the elements in the samples were investigated by X-ray photoelectron spectroscopy (XPS) using an Al Kα radiation source, with measurements performed on a PHI Quantera SXM instrument (ULVAC-PHI). The binding energies were calibrated using the C 1s peak at 284.8 eV as a reference. Diffuse reflectance spectra (DRS) were recorded using a UV-Visible spectrophotometer (V-750 equipped with an ISV-922 integrating sphere, JASCO), and photoluminescence (PL) spectra were measured using a fluorescence spectrophotometer (FP-8500, JASCO, Tokyo, Japan). Surface morphology was observed using scanning electron microscopy (SEM) with a JSM-IT700HR microscope (JEOL Ltd., Tokyo, Japan).
2.4. Photocatalytic Experiment
The photocatalytic activity was evaluated through a decolorization experiment of RhB. Specifically, 20 mg of sample was introduced into a Pyrex glass reaction vessel containing 35 mL of 10 ppm RhB. The suspension was stirred in the dark for 60 min to achieve adsorption–desorption equilibrium. Subsequently, the mixture was irradiated with a 500 W xenon lamp equipped with a cutoff filter (>420 nm), and 2 mL aliquots were collected at regular intervals. The withdrawn samples were centrifuged at 1000 rpm, and the absorbance of the resulting supernatant was measured at 554 nm using a UV-Vis spectrophotometer.
3. Results and Discussion
3.1. Crystal Structure and Morphology
The XRD patterns of BOC, BOC-N50 NB0.5, BOC-N50 KB0.5, and BOC-N50 CBx (x = 0.5, 1.0, 1.5) are shown in Figure 1a. In the pattern of pure BOC, diffraction peaks observed at 2θ = 12.94°, 23.88°, 30.13°, and 32.78° correspond to the (002), (001), (013), and (110) crystal planes of tetragonal BOC (JCPDS No. 41–1488) [7]. In addition to these diffraction peaks, BOC-N50 NB(KB)0.5 exhibit an additional peak around 10.92°, which corresponds to the (001) plane of tetragonal BiOBr (JCPDS No.73-2061) [8]. In contrast, no significant changes from BOC were observed in BOC-N50 CB1.0. This is likely due to the cationic nature of CTAB, which may hinder the effective incorporation of bromide ions.
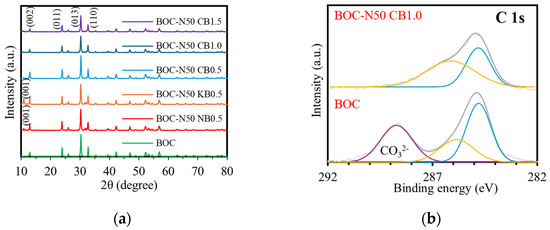
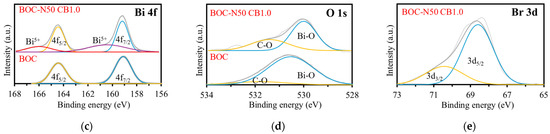
Figure 1.
(a) XRD patterns of different photocatalysts and XPS spectra of BOC and BOC-N50 CB1.0; (b) C 1s, (c) Bi 4f, (d) O 1s, (e) Br 3d.
The elemental and chemical states of BOC and BOC-N50 CB1.0 were analyzed by XPS (Figure 1b–e). Figure 1b presents the high-resolution XPS spectrum of C 1s. There were two peaks at 284.80 and 285.85 eV for BOC, and 284.80 eV and 286.12 eV for BOC-N50 CB1.0, which are classified as extrinsic carbon species in XPS measurements [9]. A carbonate-related peak at 288.72 eV is observed in BOC, but absent in BOC-N50 CB1.0, indicating partial removal of carbonate ions during nitric acid and bromine treatment. As shown in Figure 1c, peaks are detected at 159.11 eV and 164.43 eV for BOC and 159.14 eV and 164.45 eV for BOC-N50 CB1.0, which are attributed to 4f7/2 and 4f5/2, respectively [9]. These indicate that the valence state of bismuth is trivalent. Furthermore, peaks are detected at 166.40 eV and 166.01 eV for BOC-N50 CB1.0, suggesting the presence of Bi5+ species [10]. This is probably due to the oxidizing action of nitric acid, which oxidizes Bi3+ to Bi5+ and stabilizes the charge balance in the BOC lattice with the eliminated carbonate ion. In the O 1s spectrum, there are peaks at 530.54 eV and 531.79 eV for BOC and 530.0 eV and 531.40 eV for BOC-N50 CB1.0, which correspond to lattice oxygen and carbonate oxygen atoms, respectively (Figure 1d) [9]. In Figure 1e, the peaks at 68.7 eV and 70.4 eV of BOC-N50 CB1.0 were assigned to Br 3d5/2 and Br3d3/2, respectively [11].
Figure 2 presents the SEM images of the samples. All samples exhibited flower-like spherical morphologies with diameters ranging from 3 to 6 μm. Overall, no significant changes in surface morphology were observed. However, in the cases of BOC-N50 CB0.5 and BOC-N50 CB1.0, partially peeled, sheet-like features were observed compared to pure BOC, suggesting that surface etching may have occurred due to the nitric acid treatment.

Figure 2.
SEM images of (a) BOC, (b) BOC-N50 CB0.5, and (c) BOC-N50 CB1.0.
3.2. Optical Properties
Figure 3a presents the UV-Vis absorption spectra of the samples. The absorption edge of pure BOC was observed at 362 nm, whereas that of BOC-N50 CB1.0 was red-shifted to 433 nm. This red shift indicates that the treatment with nitric acid and bromine enhanced the sample’s responsiveness to visible light. Furthermore, the absorption intensity in the visible region increased with the amount of bromine added. A partial decrease in absorption around 380 nm was observed for BOC-N50 CB1.0 and BOC-N50 CB1.5, which is likely attributed to light scattering caused by residual CTAB.
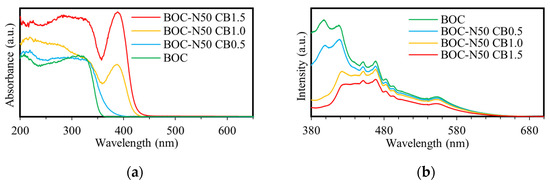
Figure 3.
(a) DRS and (b) PL spectra of different photocatalysts.
Figure 3b presents the PL spectra of the samples under an excitation wavelength of 300 nm. In general, lower fluorescence intensity indicates a reduced recombination rate of photogenerated electron–hole pairs, suggesting more efficient charge separation and enhanced charge carrier availability for photocatalytic reactions [2]. The fluorescence peak of pure BOC appears at approximately 400 nm, whereas that of BOC-N50 CB1.0 is red-shifted to around 450 nm. This red shift may be attributed to the formation of Bi5+ species and the removal of carbonate ions, which could introduce new defect levels or altered surface states, thereby affecting the carrier recombination pathways. Moreover, the lower emission intensity observed for BOC-N50 CB1.0 compared to BOC indicates enhanced separation efficiency of photogenerated charge carriers.
3.3. Photocatalytic Activity
The photocatalytic activity of the synthesized catalysts was evaluated through decolorization experiments of the dye RhB under visible light irradiation. Figure 4a presents the effect of different BOC pretreatment methods on dye decolorization. Catalysts consisting of pure BOC, acid-treated (BOC-N50), or CTAB-treated (BOC-CB0.5) exhibited negligible photocatalytic activity under visible light irradiation. This indicates that each pretreatment method alone has limited impact. In contrast, the catalyst treated with both nitric acid and CTAB (BOC-N50 CB0.5) showed significantly enhanced RhB decolorization efficiency. This improvement is attributed to the synergistic effect of acid treatment and bromine incorporation. Figure 4b presents the results of the effect of different bromine sources in the pretreatment process on RhB decolorization. Among the samples, BOC-N50 CB0.5 exhibited superior photocatalytic performance compared to BOC-N50 NB0.5 and BOC-N50 KB0.5. This enhancement is likely due to the combined effects of carbonate removal and the generation of Bi5+ species.
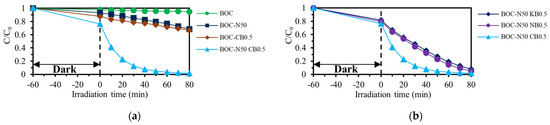
Figure 4.
Effect of (a) pretreatment method and (b) bromine sources on RhB decolorization.
Figure 5a presents the effect of different nitric acid concentrations of the pretreatment process on the photocatalytic decolorization of RhB. Compared with the catalyst treated with 10 mmol/L nitric acid, the catalysts treated with 50 mmol/L and 100 mmol/L nitric acid showed significantly enhanced initial photocatalytic activity, particularly within the first 10 min of irradiation. This improvement in initial reactivity is attributed to the stronger oxidizing power of higher nitric acid concentrations, which promotes the formation of Bi5+ species. However, the performance difference between the 50 and 100 mmol/L samples was negligible, suggesting that increasing the nitric acid concentration beyond 50 mmol/L does not yield additional benefits. Therefore, 50 mmol/L was identified as the optimal nitric acid concentration for pretreatment. Figure 5b presents the effect of different CTAB dosages of pretreatment process on the photocatalytic decolorization of RhB. Catalysts with CTAB/BOC molar ratios of 1.0 and 1.5 exhibited enhanced activity compared to the sample with a ratio of 0.5. However, the performance difference between the 1.0 and 1.5 samples was marginal, indicating that a 1:1 molar ratio of CTAB to BOC is sufficient to promote carbonate desorption and enhance photocatalytic performance.
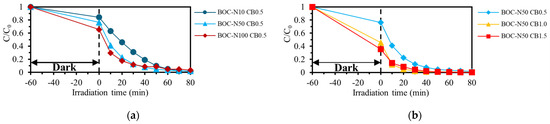
Figure 5.
Effect of (a) HNO3 concentration and (b) CTAB amount for BOC pretreatment on RhB decolorization.
Figure 6 presents the absorption spectra during RhB decolorization by BOC, BOC-N50 CB0.5, and BOC-N50 CB1.0. For the treated catalyst, the absorbance at 554 nm gradually decreased and disappeared completely within 80 min, indicating that the original dye structure had been decomposed. Additionally, the absorption peaks exhibited a blue shift, which is attributed to the deethylation of RhB [12]. Despite the peak shift, the overall absorption intensity decreased in both cases, confirming the progression of decolorization.
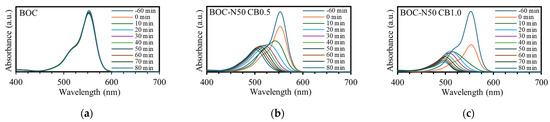
Figure 6.
Absorption spectra of photocatalytic decolorization of RhB in (a) BOC, (b) BOC-N50 CB0.5, and (c) BOC-N50 CB1.0.
3.4. Photocatalytic Mechanism
The band gap of each catalyst was estimated using the Tauc plot method, as shown in Equation (1) (Figure 7a) [11]:
where α, h, ν, A, and Eg are the absorption coefficient, Planck’s constant, light frequency, a constant, and band gap, respectively. In general, the value of n is 2 for direct band gap semiconductors and 1/2 for indirect band gap semiconductors. In this study, n = 1/2 was used for BOC. As a result, the band gap values of BOC, BOC-N50 CB0.5, BOC-N50 CB1.0, and BOC-N50 CB1.5 were estimated to be 3.42, 3.17, 2.86, and 2.82 eV, respectively. The valence band potentials (EVB, vs. NHE) of each catalyst were estimated using Equation (2), based on the vacuum level values obtained from Figure 7b [13]:
where Evs. vacuum, EVB′, and φ are the vacuum level, the value derived from VB-VPS, and the work function (4.35 eV), respectively. Evacuum of BOC, BOC-N50 CB0.5, and BOC-N50 CB1.0 were found to be 5.86, 5.43, and 6.15 V, respectively. Accordingly, the valence band potentials were calculated to be 1.42, 0.99, and 1.71 V (vs. NHE), respectively. The conduction band potentials (ECB) were subsequently estimated using Equation (3) [11]:
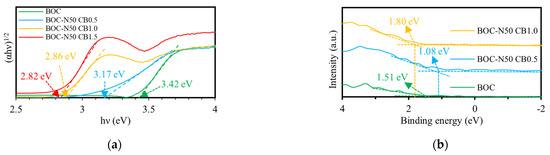
Figure 7.
(a) Tauc plots of various catalysts derived from Kubelka–Munk conversion and (b) VB-XPS spectra of the catalysts.
Based on these values, the conduction band potentials of BOC, BOC-N50 CB0.5, and BOC-N50 CB1.0 were calculated to be −2.00, −2.18, and −1.15 V (vs. NHE), respectively.
To investigate the active species in RhB decolorization by BOC-N50 CB1.0, scavenger experiments were conducted, as shown in Figure 8a. Benzoquinone (BQ), tert-butanol (TBA), and ammonium oxalate (AO) were used as scavengers for superoxide radicals (·O2−), hydroxyl radicals (·OH), and photogenerated holes (h+), respectively. BQ, TBA, and AO were added to the reaction system at concentrations of 1, 100, and 10 mmol/L, respectively. All other experimental conditions were kept constant. The addition of BQ and AO to the reaction system suppressed the decolorization efficiency of BOC-N50 CB1.0. These results suggest that ·O2− and h+ are the dominant active species responsible for RhB decolorization. Based on the above analysis, the proposed mechanism for RhB decolorization by BOC-N50 CB1.0 is shown in Figure 8b. Under visible light irradiation, electrons are excited from the valence band to the conduction band. These photogenerated electrons reduce dissolved oxygen to generate·O2−, which contributes to the decolorization of RhB. Simultaneously, h+ in the valence band directly oxidizes RhB molecules. The synergistic effect of ·O2− and h+ leads to efficient photocatalytic decolorization of RhB.
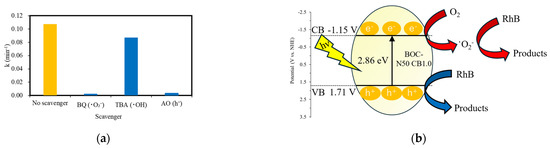
Figure 8.
(a) Effect of scavengers on RhB decolorization experiments using BOC-N50 CB1.0 and (b) photocatalytic decolorization mechanism of RhB by BOC-N50 CB1.0.
4. Conclusions
In summary, Bi2O2CO3 was treated with 50 mmol/L nitric acid and an equimolar amount of CTAB (BOC-N50 CB1.0). These treatments resulted in partial carbonate removal by the surfactant (CTAB) and partial oxidation of Bi3+ to Bi5+ by nitric acid. These modifications are believed to have reduced the recombination rate of photogenerated electron–hole pairs and narrowed the band gap. As a result, the absorbance at 554 nm in the RhB decolorization experiment gradually decreased and completely disappeared within 80 min. Moreover, a blue shift in the absorption peak was observed, while the overall absorption intensity decreased over time, confirming the progression of decolorization. Active species trapping experiments revealed that ·O2− and h+ were the primary contributors to RhB decolorization. These findings suggest that the combined treatment strategy effectively enhances the visible-light-driven photocatalytic performance of BOC.
Author Contributions
Conceptualization, H.K. and S.K.; methodology, I.T. and M.F.; validation, K.Y.; formal analysis, I.T. and M.F.; investigation, K.Y.; resources, H.K. and S.K.; data curation, H.K.; writing—original draft preparation, K.Y.; writing—review and editing, M.F.; visualization, I.T. and M.F.; supervision, S.K.; project administration, H.K.; funding acquisition, H.K. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Numbers JP18H02013, JP22H02119, JP23K23387, and JP22K14714.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deshmukh, S.A.; Suresh, S.; Bhuse, D.V.; Raut, S.U.; Reddy, M.V.B.; Ravichandran, S. Review On Strategies For the Design and Synthesis of Flower-Like Bi2WO6 and BiOX (X = F, Cl, Br, I) Composites for Photocatalytic Environmental Remediation. ChemistrySelect 2024, 9, e202401038. [Google Scholar] [CrossRef]
- Plubphon, N.; Thongtem, S.; Phuruangrat, A.; Randorn, C.; Kaowphong, S.; Thongtem, T. Rapid Preparation of G-C3N4/Bi2O2CO3 Composites and Their Enhanced Photocatalytic Performance. Diam. Relat. Mater. 2022, 130, 109488. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, T.; Nie, Z.; Wu, Q.; Huang, Y.; Zheng, M. In-Situ Construction of 3D Hollow Bi0/Bi2O2CO3 Heterojunction with Rich Oxygen Vacancies for Photocatalytic Degradation of Two Typical Pollutants in Mineral Processing Wastewater. J. Alloys Compd. 2025, 1011, 178405. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Pan, L.; Wen, Z.; Yao, S. Designing Manganese-Doped Bi/Bi2O2CO3 Microspheres for Improved Visible-Light-Induced Degradation. J. Phys. Chem. Solids 2025, 200, 112625. [Google Scholar] [CrossRef]
- Li, Y.; Ai, L.; Sheng, R.; Tan, C.; Zha, M.; Jia, D.; Guo, N.; Wang, L. In Situ Constructing 2D/2D Layered BiOBr/Bi2O2CO3 Heterostructure for Efficient Photocatalytic Reduction CO2 to CO. J. Mol. Liq. 2024, 413, 125960. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Wang, F.; Cao, K.; Doronkin, D.E.; Dong, F.; Grunwaldt, J.-D. Facile Synthesis of Surface N-Doped Bi2O2CO3: Origin of Visible Light Photocatalytic Activity and in Situ DRIFTS Studies. J. Hazard. Mater. 2016, 307, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Ma, Y.; Shang, Y.; Tan, P.; Pan, J. Self-Integrated β-Bi2O3/Bi2O2.33@Bi2O2CO3 Ternary Composites: Formation Mechanism and Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 613–624. [Google Scholar] [CrossRef]
- Bi, F.; Zheng, Z.; Li, R.; Du, R.; Zhao, L.; Xiao, S.; Wang, L.; Dong, X. Design and Performance Investigation of Novel Efficient Photocatalysts PVP-Modified PVDF/BiOBr Photocatalytic Membranes for Wastewater Treatment. Chem. Eng. J. 2025, 507, 160781. [Google Scholar] [CrossRef]
- Sun, D.; Huang, L.; Li, L.; Yu, Y.; Du, G.; Xu, B. Plasma Enhanced Bi/Bi2O2CO3 Heterojunction Photocatalyst via a Novel in-Situ Method. J. Colloid. Interface Sci. 2020, 571, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, L.; Zhang, Y.; Wei, Y. Bi5+, Bi(3−x)+, and Oxygen Vacancy Induced BiOClx I1−x Solid Solution toward Promoting Visible-Light Driven Photocatalytic Activity. Chem. A Eur. J. 2018, 24, 7434–7444. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Cao, J.; Lin, H.; Zhang, M.; Guo, X.; Chen, S. Transforming Type-I to Type-II Heterostructure Photocatalyst via Energy Band Engineering: A Case Study of I-BiOCl/I-BiOBr. Appl. Catal. B 2017, 204, 505–514. [Google Scholar] [CrossRef]
- Lei, X.; Hu, S.; Liu, K.; Lv, X.; Chen, Y.; Zhang, Q.; Jia, Y.; Zhong, K.; Wang, B.; Xu, T. Electrochemical Oxidation of Rhodamine B in Dye Wastewater by a Novel Boron-Doped Diamond Electrode: Parameter Optimization and Degradation Mechanism. Desalination Water Treat. 2024, 317, 100243. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Qin, Q.; Huang, C.; Niu, Y.; Li, M.; Song, B.; Fan, B.; Shao, G.; Lu, H.; et al. Significantly Improvement of the Photocatalytic Performance of Vermiculite/g-C3N4 Composite by the Modification of BiOBr. Mater. Chem. Phys. 2024, 322, 129550. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).