Isolation and Characterization of Two Coumarin Compounds from the Chloroform Fraction of Scadoxus multiflorus (Martyn) Raf. (Amaryllidaceae) †
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Extraction and Partitioning
2.3. Isolation and Characterization of Compound B12 and C11 from Chloroform Fraction
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense Against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.A.; Babalola, O.O. Secondary Metabolites as Plant Defensive Strategy: A Large Role for Small Molecules in the Near Root Region. Planta 2020, 252, 61. [Google Scholar] [CrossRef]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and Pharmacological Profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.M.A.E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; IntechOpen: London, UK, 2015; pp. 113–140. [Google Scholar] [CrossRef]
- Mim, B.A.; Md Mashrur, C.; Rajib, H.; Tania, P.; Fatema, K.; Anzuman, A.; Islam, M.T.; Razina, R. Phyto-pharmacological Investigation of Aqueous Crude Extract of Scadoxus multiflorus. World J. Pharm. Pharm. Sci. 2021, 10, 154–173. [Google Scholar] [CrossRef]
- Yokosuka, A.; Suzuki, N.; Mimaki, Y. Chemical constituents of the bulbs of Haemanthus multiflorus. Phytochem. Lett. 2017, 21, 6–10. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Royal Botanical Gardens: Kew, UK, 1985; Volume 1, p. 319. [Google Scholar]
- Tsukamoto, K. Juveniles Released with Fluorescent Otolith Tags. News Bay 1989, 35, 59–69. [Google Scholar]
- Alhakmani, F.; Kumar, S.; Alam Khan, S. Estimation of Total Phenolic Content, In-Vitro Antioxidant and Anti–Inflammatory Activity of Flowers of Moringa Oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, B.V.; Kadhirvelu, P.; Nadipelly, J.; Shanmugasundaram, J.; Sayeli, V.; Subramanian, V. Anti-nociceptive Effect of 7-methoxy Coumarin from Eupatorium triplinerve vahl (Asteraceae). Pharmacogn Mag. 2017, 13, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Chai, H.; Farnsworth, N.I.; Gupta, M.P. Natural Product Isolation; Carnell, R., Ed.; Humana Press Publication: Clifton, NJ, USA, 1998; pp. 349–359. [Google Scholar]
- Bose, P.K.; Roy, A.C. The Constitution of Ayapanin. J. Indian Chem. Soc. 1936, 13, 586–587. [Google Scholar]
- Natarajan, R.K.; Natarajan, M.J. Phytochemical Investigation of Eupatorium Ayapana. J. Res. Indian Med. Yoga Homeopat. 1979, 14, 155–156. [Google Scholar]
- Xiu, C.; Hua, Z.; Xiao, B.; Tang, W.J.; Zhou, H.P.; Liu, X.H. Expert Opinion on Therapeutic Patents Novel Benzopyran Derivatives and their Therapeutic Applications: A Patent Review. Expert Opin. Ther. Pat. 2017, 00, 1–15. [Google Scholar] [CrossRef]
- Budavári, T.; Szalay, A.S.; Charlot, S.; Seibert, M.; Wyder, T.K.; Arnouts, S.; Barlow, T.A.; Bianchi, L.; Byun, Y.-I.; Donas, J.; et al. The Ultraviolet Luminosity Function of Galex Galaxies at Photometric Redshifts between 0.07 and 0.25. Astrophys. J. 2005, 619, L31–L34. [Google Scholar] [CrossRef]
- Lide, D.R.; Milne, G.W. Properties of Organic Compounds. Pure Appl. Chem. 1996, 55, 221–228. [Google Scholar]
- Netea, M.G.; Demacker, P.N.; Kullberg, B.J.; Boerman, O.C.; Verschueren, I.; Stalenhoef, A.F.; van der Meer, J.W. Low-Density Lipoprotein Receptor-Deficient Mice Are Protected Against Lethal Endotoxemia and Severe Gram-Negative Infections. J. Clin. Investig. 1996, 97, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Raunio, H.; Pentikäinen, O.; Juvonen, R.O. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 4708. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, A.; Barrachina, I.; El Aouad, N.; Franck, X.; Chahboune, N.; Andreu, I.; Figadère, B.; Vila, L.; Hennuyer, N.; Staels, B.; et al. Synthesis of Benzopyran Derivatives as PPARα and/or PPARγ activators. Bioorg. Med. Chem. 2019, 27, 115162. [Google Scholar] [CrossRef] [PubMed]
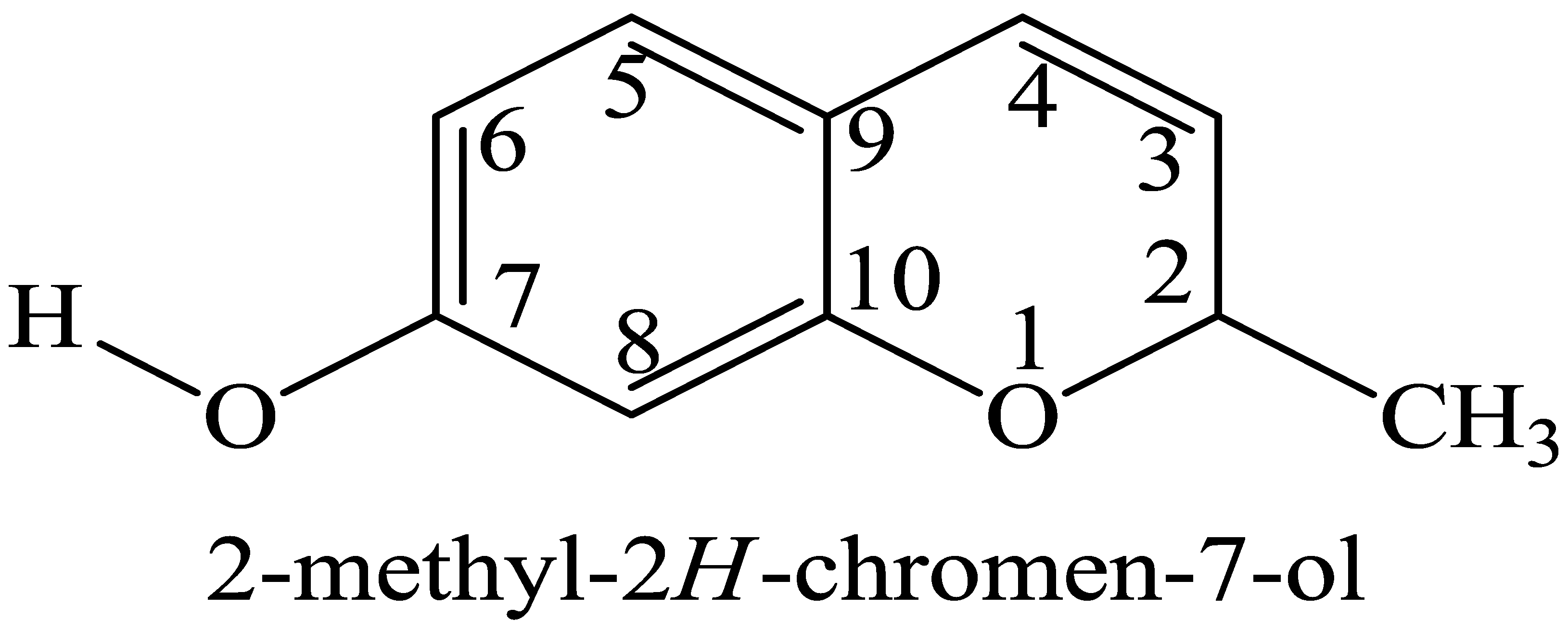
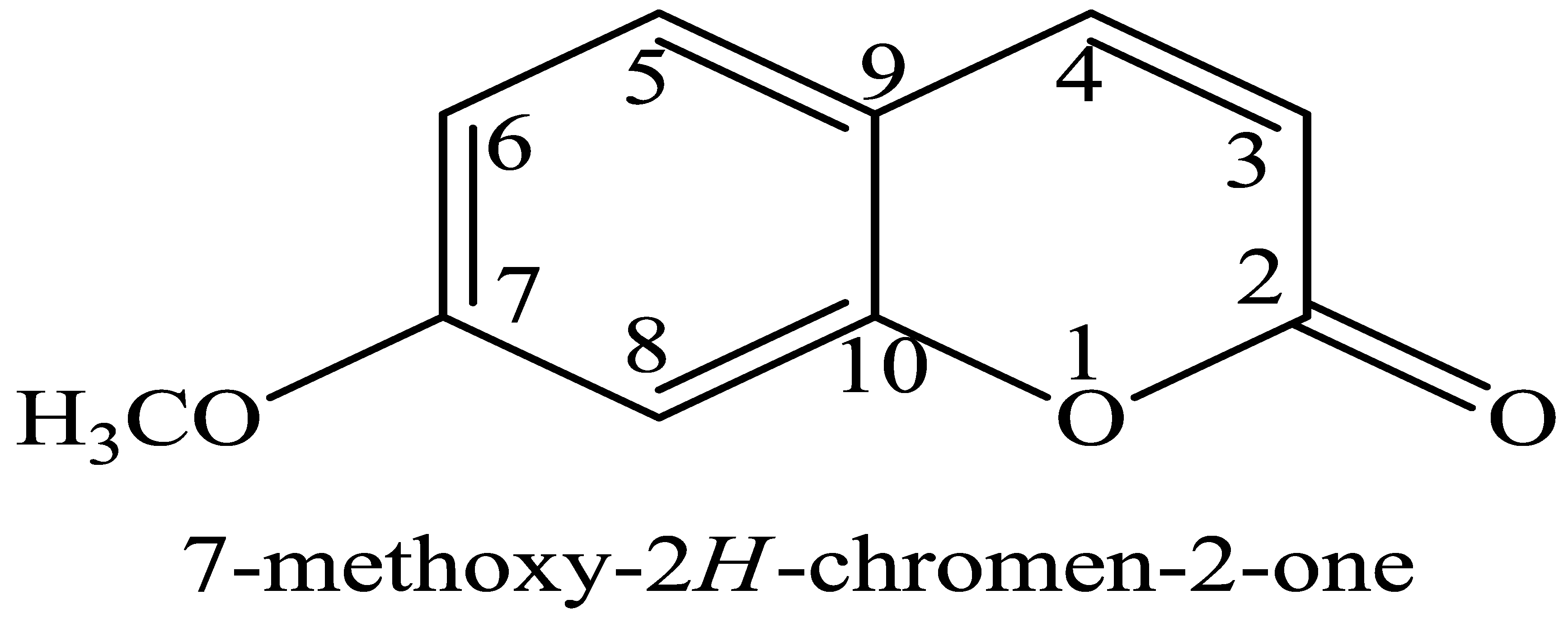
| Compound B12 | Compound C11 | ||||
|---|---|---|---|---|---|
| C | δH (ppm) | δC (ppm) | C | δH (ppm) | δC (ppm) |
| 1 | ----- | ----- | 1 | ----- | ----- |
| 2 | 4.09 (s) | 56.79 | 2 | ----- | 167.04 |
| 3 | 6.89 (d, J = 4.0 Hz) | 108.15 | 3 | 6.90 (d, J = 4.0 Hz) | 118.73 |
| 4 | 8.05 (t, J = 4.0, 8.0 Hz) | 124.68 | 4 | 8.02 (t, J = 4.0, 8.0 Hz) | 124.01 |
| 5 | 7.95 (t, J = 4.0, 8.0 Hz) | 118.73 | 5 | 7.93 (t, J = 4.0, 8.0 Hz) | 130.28 |
| 6 | 7.46 (d, J = 4.0 Hz) | 110.45 | 6 | 7.49 (d, J = 4.0 Hz) | 110.45 |
| 7 | OH | 129.32 | 7 | ----- | 160.11 |
| 8 | 7.75 (s) | 111.42 | 8 | 7.72 (s) | 111.43 |
| 9 | ----- | 116.72 | 9 | ----- | 116.43 |
| 10 | ----- | 151.16 | 10 | ----- | 152.16 |
| CH3 | 0.86 (s) | 18.08 | OCH3 | 3.69 (s) | 55.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayodele, O.A.; Omolara, T.T.; Musa, A.S.; Ibrahim, S.M. Isolation and Characterization of Two Coumarin Compounds from the Chloroform Fraction of Scadoxus multiflorus (Martyn) Raf. (Amaryllidaceae). Chem. Proc. 2024, 16, 89. https://doi.org/10.3390/ecsoc-28-20184
Ayodele OA, Omolara TT, Musa AS, Ibrahim SM. Isolation and Characterization of Two Coumarin Compounds from the Chloroform Fraction of Scadoxus multiflorus (Martyn) Raf. (Amaryllidaceae). Chemistry Proceedings. 2024; 16(1):89. https://doi.org/10.3390/ecsoc-28-20184
Chicago/Turabian StyleAyodele, Olaiya Akeem, Tijani Tawakaltu Omolara, Abdullahi Sakynah Musa, and Sule Mohammed Ibrahim. 2024. "Isolation and Characterization of Two Coumarin Compounds from the Chloroform Fraction of Scadoxus multiflorus (Martyn) Raf. (Amaryllidaceae)" Chemistry Proceedings 16, no. 1: 89. https://doi.org/10.3390/ecsoc-28-20184
APA StyleAyodele, O. A., Omolara, T. T., Musa, A. S., & Ibrahim, S. M. (2024). Isolation and Characterization of Two Coumarin Compounds from the Chloroform Fraction of Scadoxus multiflorus (Martyn) Raf. (Amaryllidaceae). Chemistry Proceedings, 16(1), 89. https://doi.org/10.3390/ecsoc-28-20184





