Abstract
This study investigated the role of silver nanoparticles (AgNP) in the inhibition of fecal coliforms by 3D-printed modified chitosan filtration membranes. The composite membranes demonstrated steady improvement in antibacterial activity against the bacteria. The amount of silver ions (Ag+) added to chitosan/AgNP filtration membranes affectshow well they kill microbes. An increase in the concentration of AgNP improved the chitosan matrix’s antibacterial activity and effectively reduced fecal coliforms. However, only fecal microorganisms in contact with Ag+ experienced complete destruction or inhibition from the modified composite membranes. The membrane surface structural layer revealed that the CS/AgNP composite consisted of carbon (C), oxygen (O), silver (Ag), and small amounts of sulfur (S). When active fecal bacteria cells came into contact with the CS/AgNP membrane structure, it was able to break down their barrier properties. The positively charged sites of the modified chitosan matrix effectively interacted with negatively charged microbial cells and eventually reduced fecal activities by 99.9%. The measured Ag concentrations in the effluent decreased over a period of time, suggesting that an increase in the volume of effluent would bring about a reduction in the concentration of Ag ions. Therefore, optimizing the amount of Ag nanoparticles in the modified chitosan composite is necessary to achieve the most favorable membrane separation performance for treating polluted surface water.
1. Introduction
The provision of safe drinking water is a critical component of achieving sustainable development. Nevertheless, the presence and rapid growth of microbiological contaminants in surface water have consistently posed a significant threat to human well-being and have emerged as a prominent societal concern. The primary contributor of fecal bacteria, including pathogens, to surface water is the release of waste from human and animal feces. This has led to the emergence of several waterborne illnesses due to the presence of microorganisms. The bacteria present in drinking water are derived from the gastrointestinal tracts of endothermic organisms. The sources of pollution include several factors, including animals, pets, and inadequately designed or malfunctioning waste treatment facilities, such as privately owned septic systems and sewage pipes. Therefore, it is essential to engage in regular surveillance of fecal contamination in surface water in order to safeguard public health [1]. Fecal indicator bacteria, including coliforms and E. coli, have been used for the purpose of evaluating fecal contamination in surface water.
2. Materials and Methods
2.1. Materials
Medium-molecular-weight Chitosan (198 KDa) with more than 75% degree of deacetylation was procured from Shandong, China. Merck (Sigma-Aldrich) Life Science Johannesburg, South Africa supplied 99% silver nitrate (AgNO3), polyvinyl alcohol (PVA) with a molecular weight of 22 kDa (88% hydrolysis degree), and 98% sodium hydroxide (NaOH). Glassworld Johannesburg, South Africa provided distilled water (H2O), 98% sulfuric acid (H2SO4), glacial acetic acid, and isopropyl alcohol (C3H8O). Lab-Chem (Johannesburg, South Africa) provided trisodium citrate (C6H5O3Na3). All the chemicals employed were of analytical grade and were not further purified.
2.2. Preparation and Inkjet Printing of Chitosan/AgNP Composite
Silver nanoparticles were prepared by the direct reduction of silver nitrate by trisodium citrate (C6H5O3Na3) using a modified Tollens’ process [2]. Chitosan solution was prepared by dissolving 2 g of CS in 2% acetic acid under continuous agitation for 12 h, left for 6 h to ensure that all air pockets were eliminated, and further treated with buffer solution to adjust its pH to neutral. A Colorsun 1630 UV (Shenzhen, China) with an Epson L805 drop-on-demand piezoelectric printhead inkjet printer was used for the fabrication of the modified CS composite, while PVA solution was employed as co-solvent in the ink formulation. The printed membranes were further cross-linked in a solution containing 0.01 M H2SO4 for 30 min, rinsed several times in distilled water, and preserved in desiccators to avoid contamination.
2.3. Characterization
The characterization of microbiological analysis of the modified chitosan composite was done by Ambio Laboratories-VUT, and reagents were prepared in accordance with the protocol utilized in previous studies [3]. The surface morphology of composite samples was examined using a Field Emission Scanning Electron Microscope (FESEM)-JSM-7900F (Tokyo, Japan) in conjunction with an energy-dispersive X-ray spectrometer(EDS).
2.4. Antibacterial Study
The fecal inhibition was conducted using a filtering system with a vacuum pump and running at a constant pressure of 220 mmHg. An agar plate technique was adopted for the antibacterial study of the CS/AgNP composite according to the established method [4]. Briefly, 100 mL of contaminated water samples were filtered through the modified CS/AgNP nanocomposite membrane samples placed on absorbable 0.45 μm grid membrane filter sheets. The membrane filter sheets were then placed on sterile Petri dishes impregnated with MFC agar. The agar plates were incubated at 37 °C for 24 h. After the incubation period, the agar plates were removed from the incubator, the number of bacterial colonies was counted using microscopy, and the number of colony-forming units (CFU) per 100 mL sample was calculated using the standard equation (Equation (1)).
Number of CFU per 100 mL = (Number of colonies on the membrane)/(volume of sample filtered) × 100
3. Results and Discussion
3.1. Effects of AgNP Concentration on Fecal Inhibition
According to CFU/100 mL calculations, about 10,000 fecal coliforms were detected in the polluted water sample. Table 1 demonstrates that the modified CS/AgNP composite membranes had a lower number of fecal colonies than the positive control. Sample 1 contained 120 CFU/100 mL of fecal coliforms, while samples 2 and 3 contained 110 CFU/100 mL and 89 CFU/100 mL, respectively. This trend may be attributable to the enhanced antibacterial activity of modified CS membrane composites.

Table 1.
Summary of fecal inhibition performance.
Consequently, an increase in the amount of Ag in the membrane composite might be responsible for the decline in fecal activity. Also, the ratio of fecal concentration in the effluent to that in the influent decreased substantially across all samples: S1 (10,000:120), S2 (10,000:110), and S3 (10,000:89). The average fecal concentration reduced as follows: 98.8% (S1), 98.9% (S2), and 99.1% (S3).
Inductively coupled plasma mass spectrometry (ICP-MS) was used to assess the concentration of silver ions in the effluent samples. Figure 1 depicts the comparative analysis of the fecal concentration detected in the effluent after 3 h of effluent discharge to demonstrate the relative inhibition strength of the membrane composite samples. The concentration of fecal coliforms decreased as the amount of Ag in the CS/AgNP composite increased gradually. Sample 3 exhibited the highest concentration of Ag (0.0315 mL/L) with the lowest fecal concentration (89.0 CFU/100 mL) detected in the filtrate, whereas sample 1 had the lowest inhibition of fecal coliforms (120 CFU/100 mL) with the lowest concentration of Ag ions (0.0237 mL/L) in the effluent.
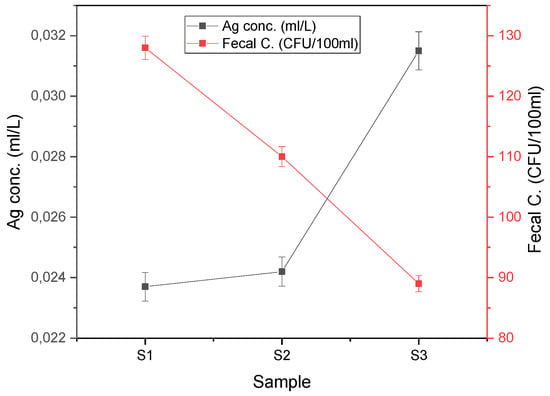
Figure 1.
Effects of Ag concentration on fecal inhibition.
3.2. Half-Hourly Concentration of Ag Ions in Effluent Discharge
As shown in Table 2, the concentration of silver ions in the effluent discharge decreased progressively over a period of time. This could suggest that the effluent contains traces of Ag ions. The measured total Ag concentrations in the effluent are within acceptable limits for drinking water according to the World Health Organization [5]. Therefore, the modified CS filtration membranes can be utilized for water treatment. The Ag concentrations in the effluent decreased over a period of time in the following order: S3 > S2 > S1. Figure 2 illustrates the half-hourly decline in the concentration of Ag+ in the effluent samples. This could suggest that an increase in the volume of effluent (filtrates) would bring about a reduction in the concentration of Ag ions in the effluent.

Table 2.
Summary of fecal inhibition performance.
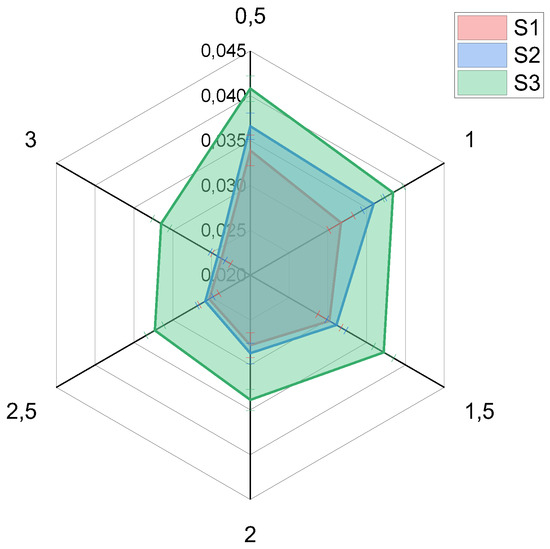
Figure 2.
Half-hourly reduction in Ag ion concentration.
The surface layered image structure of the printed membrane composite is depicted in Figure 3. Clearly, the membrane surface is not homogenous due to the layer-by-layer polymerization procedure, unlike conventional membranes which typically have uniform surface morphologies [6]. The surface structural layer also revealed various elemental components in the CS/AgNP composite, including carbon (C), oxygen (O), silver (Ag), and traces of sulfur (S), which are highlighted in different colors across the membrane surface according to the energy-dispersive X-ray spectroscopy analysis (EDS).The scattered blue colors across the membrane surface indicate an asymmetrical distribution of Ag nanoparticles across the surface of the modified CS membrane. Consequently, regions with a higher intensity of blue colors would indicate a higher concentration of Ag nanoparticles with greater membrane antimicrobial performance [7].
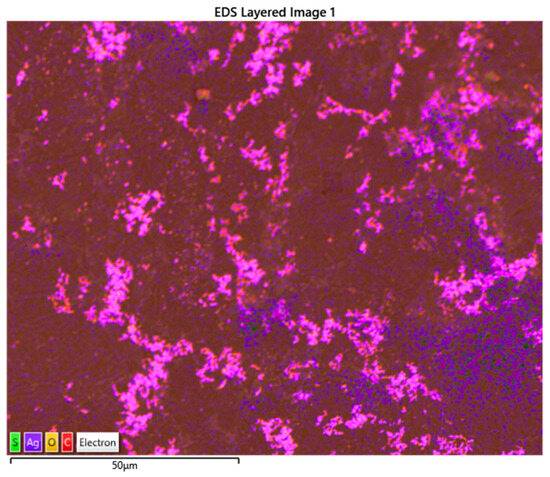
Figure 3.
EDS layered structure of CS/AgNP composite.
3.3. Mechanisms of CS-AgNP Antimicrobial Activities
Figure 4 depicts the proposed antibacterial activities of the CS/AgNP-modified composite. The mechanism was proposed in two separate phases. The first stage ‘A’ demonstrates the attachment of Ag to the structural network of CS, while the second stage ‘B’ demonstrates antibacterial activity against fecal coliform bacteria strains in contaminated water via membrane nanofiltration. The CS/AgNP membrane was capable of disrupting the barrier properties of each active fecal bacteria cell that came into contact with the composite [8]. It is possible that positively charged sites of the modified chitosan matrix could interact with negatively charged microbial cells and eventually reduces fecal activities [9]. Only those fecal microorganisms that came into direct contact with Ag ions would be completely eliminated, while the rest would merely be weakened or survived. A higher concentration of AgNP would increase the number of Ag+ and, consequently, the number of fecal microorganisms that are killed or inactivated during the filtration process.
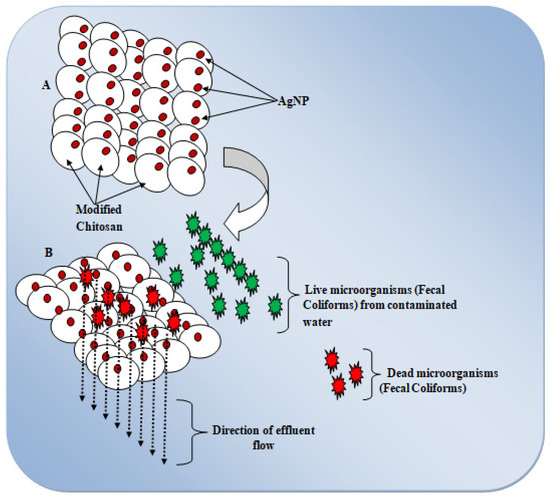
Figure 4.
Proposed antimicrobial mechanisms of CS/AgNP composite.
4. Conclusions
The effectiveness of chitosan/AgNP filtration membranes in combating and eradicating microbial activities depends on the concentration of Ag doped into the membrane composite. Fecal coliform concentration declined with a gradual increase in the amount of Ag nanoparticles within the chitosan matrix. However, only fecal microorganisms in contact with Ag+ were completely destroyed or inhibited by the modified chitosan composite membranes.
Author Contributions
A.C.O. conducted the experiments and wrote the manuscript; P.O.O. supervised the entire work and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Vaal University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the technical assistance and financial support provided by the Chemical Industries Education & Training Authority (CHIETA), and the Faculty of Engineering and Technology, Vaal University of Technology, Gauteng.
Conflicts of Interest
The authors declare no competing interest.
References
- World Health Organization. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003.
- Faria, A.F.; Liu, C.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosis membranes functionalized with graphene oxide–silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Ogazi, A.C.; Osifo, P.O. Inhibition of coliforms and Escherichia coli bacterial strains in water by 3D printed CS/GO/AgNP filtration membranes. J. Polym. Environ. 2023, 31, 4448–4467. [Google Scholar] [CrossRef]
- Gu, B.; Jiang, Q.; Luo, B.; Liu, C.; Ren, J.; Wang, X.; Wang, X. A sandwich-like chitosan-based antibacterial nanocomposite film with reduced graphene oxide immobilized silver nanoparticles. Carbohydr. Polym. 2021, 260, 117835. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition; World Health Organization: Geneva, Switzerland, 2017.
- Chowdhury, M.R.; Steffes, J.; Huey, B.D.; McCutcheon, J.R. 3D printed polyamide membranes for desalination. Science 2018, 361, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Marta, B.; Potara, M.; Iliut, M.; Jakab, E.; Radu, T.; Imre-Lucaci, F.; Katona, G.; Popescu, O.; Astilean, S. Designing chitosan–silver nanoparticles–graphene oxide nanohybrids with enhanced antibacterial activity against Staphylococcus aureus. Colloids Surf. A Physicochem. Eng. Asp. 2015, 487, 113–120. [Google Scholar] [CrossRef]
- Wang, L.S.; Wang, C.Y.; Yang, C.H.; Hsieh, C.L.; Chen, S.Y.; Shen, C.Y.; Wang, J.J.; Huang, K.S. Synthesis and anti-fungal effect of silver nanoparticles–chitosan composite particles. Inter. J. Nanomed. 2015, 10, 2685–2696. [Google Scholar]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).