Regioselective Synthesis of Coumarin-Annulated Polycyclic Heterocycles via Sequential Claisen Rearrangement and Radical Cyclization Reaction †
Abstract
1. Introduction
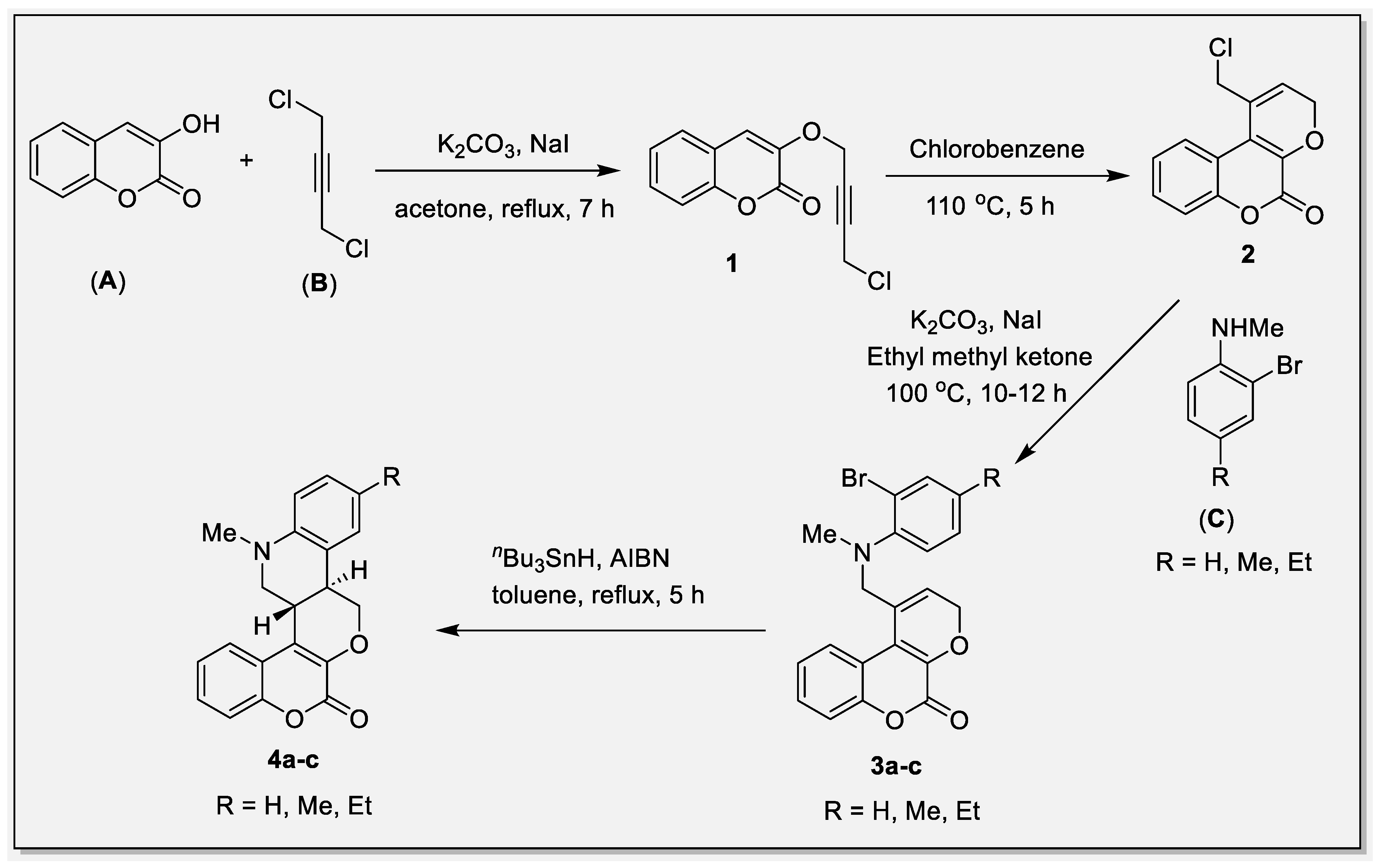
2. Results and Discussion
3. Conclusions
4. Experimental
- (a)
- General procedure for the radical cyclization of substrates 3 to cyclized product 4.
- (b)
- Spectral analysis:
- 1-(((2-Bromophenyl)(methyl)amino)methyl)pyrano[2,3-c]chromen-5(3H)-one (3a)

- 1-(((2-Bromo-4-methylphenyl)(methyl)amino)methyl)pyrano[2,3-c]chromen-5(3H)-one (3b)
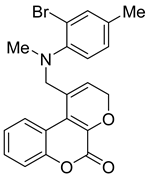
- 1-(((2-Bromo-4-ethylphenyl)(methyl)amino)methyl)pyrano[2,3-c]chromen-5(3H)-one (3c)
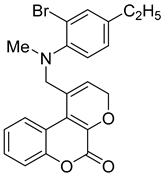
- (6cR,12bR)-8-methyl-7,8,12b,13-tetrahydrochromeno[4′,3′:5,6]pyrano[4,3-c]quinolin-1(6cH)-one (4a)
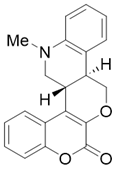
- (6cR,12bR)-8,11-Dimethyl-7,8,12b,13-tetrahydrochromeno[4′,3′:5,6]pyrano[4,3-c]quinolin-1(6cH)-one (4b)

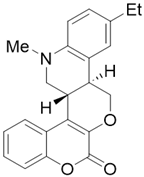
- (6cR,12bR)-11-ethyl-8-methyl-7,8,12b,13-tetrahydrochromeno[4′,3′:5,6]pyrano[4,3-c]quinolin-1(6cH)-one (4c)
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Liao, Y.; Yang, Z. Synthesis of 4-substituted coumarins via the palladium catalyzed cross-coupling of 4-tosylcoumarins with terminal acetylenes and organozinc reagents. J. Org. Chem. 2001, 66, 3642. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure–Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073. [Google Scholar] [CrossRef] [PubMed]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and Biological Properties of Coumarin Derivatives. A Review. ChemistrySelect 2021, 6, 5848. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Murakami, A.; Gao, G.; Omura, M.; Yano, M.; Ito, C.; Furukawa, H.; Takahasi, D.; Koshimizu, K.; Ohigashi, H. 1,1-Dimethylallylcoumarins potently supress both lipopolysaccharide- and interferon-g-induced nitric oxide generation in mouse macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2000, 10, 59. [Google Scholar] [CrossRef]
- Guilet, D.; Helesbeux, J.J.; Seraphin, D.; Sevenet, T.; Richomme, P.; Bruneton, J. Novel cytotoxic 4-phenylfuranocoumarins from Calophyllum dispar. J. Nat. Prod. 2001, 64, 563. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological Properties of Coumarins. Mini Rev. Med. Chem. 2016, 18, 113–141. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Natural Products as Fungicide and Their Role in Crop Protection. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A., Eds.; Springer: Singapore, 2020; pp. 131–219. [Google Scholar]
- Azim, S.A.; Al-Hazmy, S.M.; Ebeid, E.M.; El-Daly, S.A. A new coumarin laser dye 3-(benzothiazol-2-yl)-7-hydroxycoumarin. Opt. Laser Technol. 2005, 37, 245–249. [Google Scholar] [CrossRef]
- Gandioso, A.; Contreras, S.; Melnyk, I.; Oliva, J.; Nonell, S.; Velasco, D.; García-Amorós, J.; Marchán, V. Development of green/red-absorbing chromophores based on a coumarin scaffold that are useful as caging groups. J. Org. Chem. 2017, 82, 5398–5408. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Liu, T.; Sun, J.; Wang, X.-J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Khan, D.; Shaily. Coumarin-based fluorescent sensors. Appl. Organomet. Chem. 2023, 37, e7138. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Nandi, R.K. The Claisen rearrangement in the syntheses of bioactive natural products. Tetrahedron 2013, 69, 6921–6957. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Alam, S.; Chattopadhyay, B. Catalysis of the Claisen rearrangement. Tetrahedron 2008, 64, 597–643. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Debnath, P.; Maji, P.K. Rradical mediated cyclization reaction: Regioselective synthesis of pyrimidine and coumarin-annulated [6,6]fused oxygen heterocycles. Can. J. Chem. 2008, 86, 846–854. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Saha, D.; Debnath, P. Studies on sequential Claisen rearrangement: Charge-accelerated [3,3]-sigmatropic rearrangement leading to polyheterocycles. Synth. Commun. 2007, 37, 3657–3665. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Debnath, P.; Chottopadhya, S.K. Aryl Radical cyclization: Regioselective synthesis of 6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]quinolin-6-one. Synth. Commun. 2008, 38, 1768–1777. [Google Scholar] [CrossRef]
- Liao, J.; Yang, X.; Ouyang, L.; Lai, Y.; Huang, J.; Luo, R. Recent advances in cascade radical cyclization of radical acceptors for the synthesis of carbo- and heterocycles. Org. Chem. Front. 2021, 8, 1345–1363. [Google Scholar] [CrossRef]
- Yu, X.-C.; Zhang, C.-C.; Wang, L.-T.; Li, J.-Z.; Li, T.; Wei, W.-T. The synthesis of seven- and eight-membered rings by radical strategies. Org. Chem. Front. 2022, 9, 4757. [Google Scholar] [CrossRef]
- Romero, K.J.; Galliher, M.S.; Pratt, D.A.; Stephenson, C.R.J. Radicals in natural product synthesis. Chem. Soc. Rev. 2018, 47, 7851–7866. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debnath, P. Regioselective Synthesis of Coumarin-Annulated Polycyclic Heterocycles via Sequential Claisen Rearrangement and Radical Cyclization Reaction. Chem. Proc. 2024, 16, 87. https://doi.org/10.3390/ecsoc-28-20127
Debnath P. Regioselective Synthesis of Coumarin-Annulated Polycyclic Heterocycles via Sequential Claisen Rearrangement and Radical Cyclization Reaction. Chemistry Proceedings. 2024; 16(1):87. https://doi.org/10.3390/ecsoc-28-20127
Chicago/Turabian StyleDebnath, Pradip. 2024. "Regioselective Synthesis of Coumarin-Annulated Polycyclic Heterocycles via Sequential Claisen Rearrangement and Radical Cyclization Reaction" Chemistry Proceedings 16, no. 1: 87. https://doi.org/10.3390/ecsoc-28-20127
APA StyleDebnath, P. (2024). Regioselective Synthesis of Coumarin-Annulated Polycyclic Heterocycles via Sequential Claisen Rearrangement and Radical Cyclization Reaction. Chemistry Proceedings, 16(1), 87. https://doi.org/10.3390/ecsoc-28-20127





