Cobalt (II) Complex on Nanodiamond-Grafted Polyethyleneimine@Folic Acid: An Extremely Effective Nanocatalyst for Green Synthesis of 5-Substituted 1H-Tetrazole Derivatives †
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Apparatus
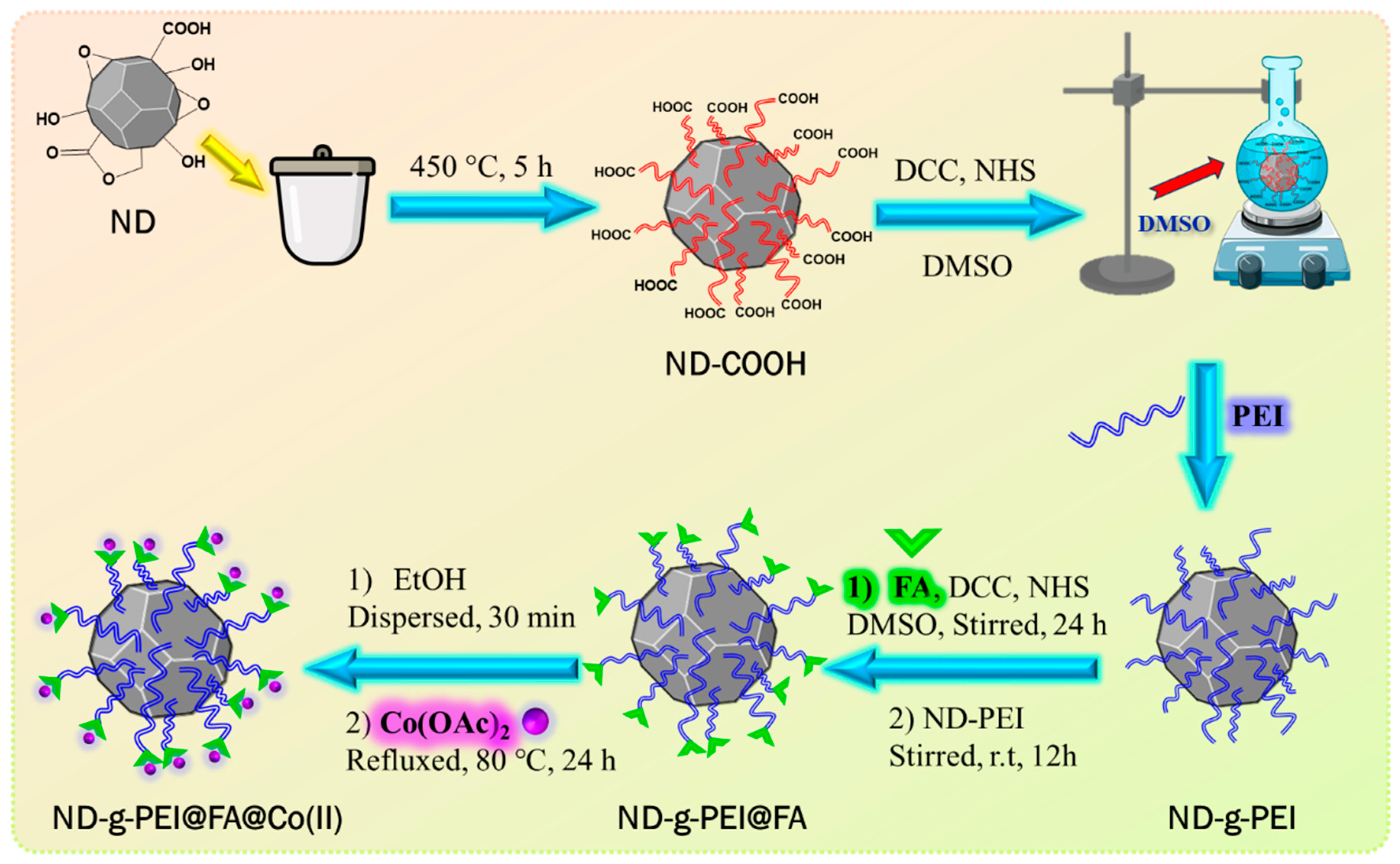
2.2. Preparation of ND-g-PEI
2.3. Preparation of ND-g-PEI@FA
2.4. Preparation of ND-g-PEI@FA@Co(II)
2.5. General Procedure for the Synthesis of 5-Substituted 1H-Tetrazole Derivatives
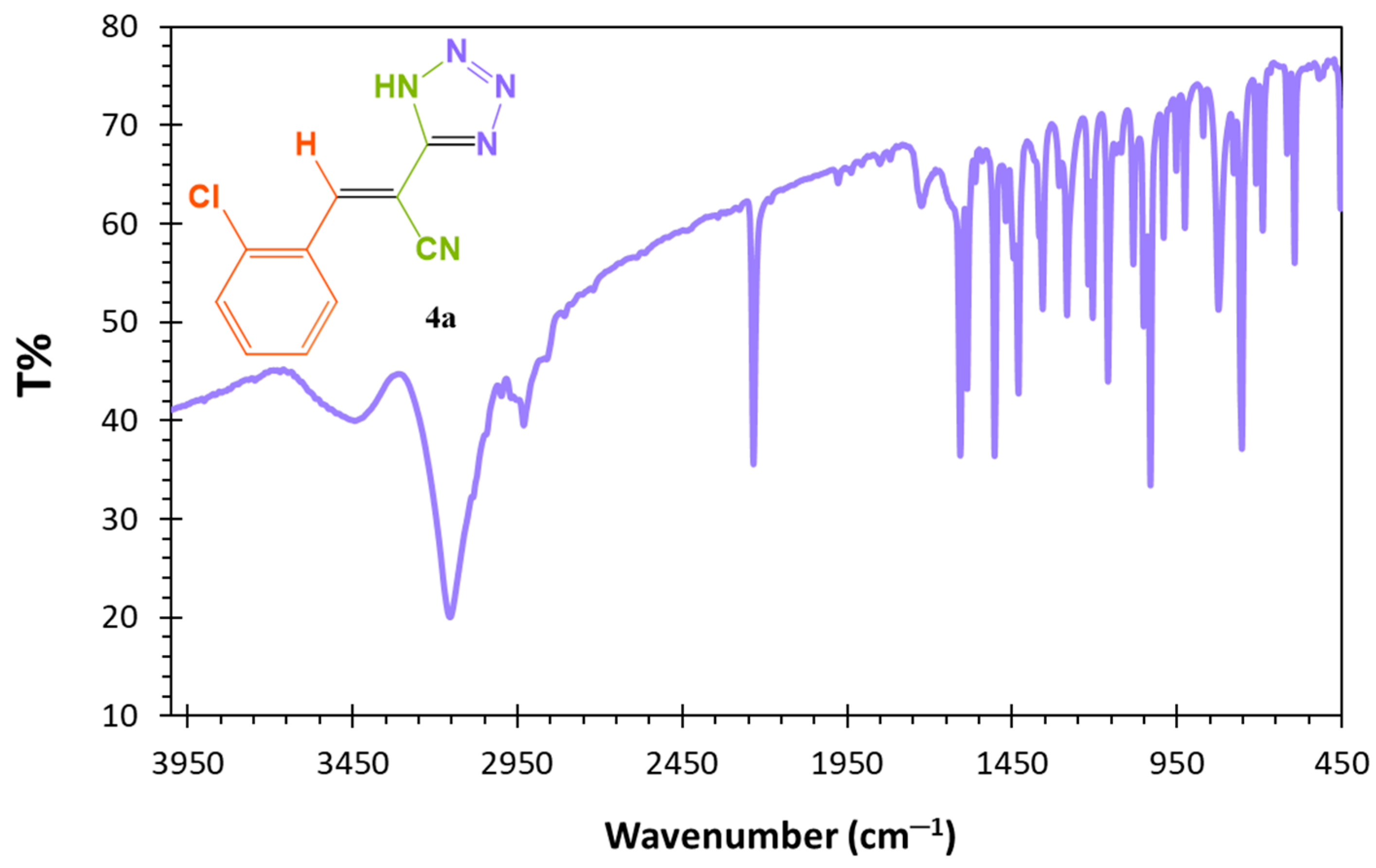
2.6. Spectral Data
3. Results and Discussion
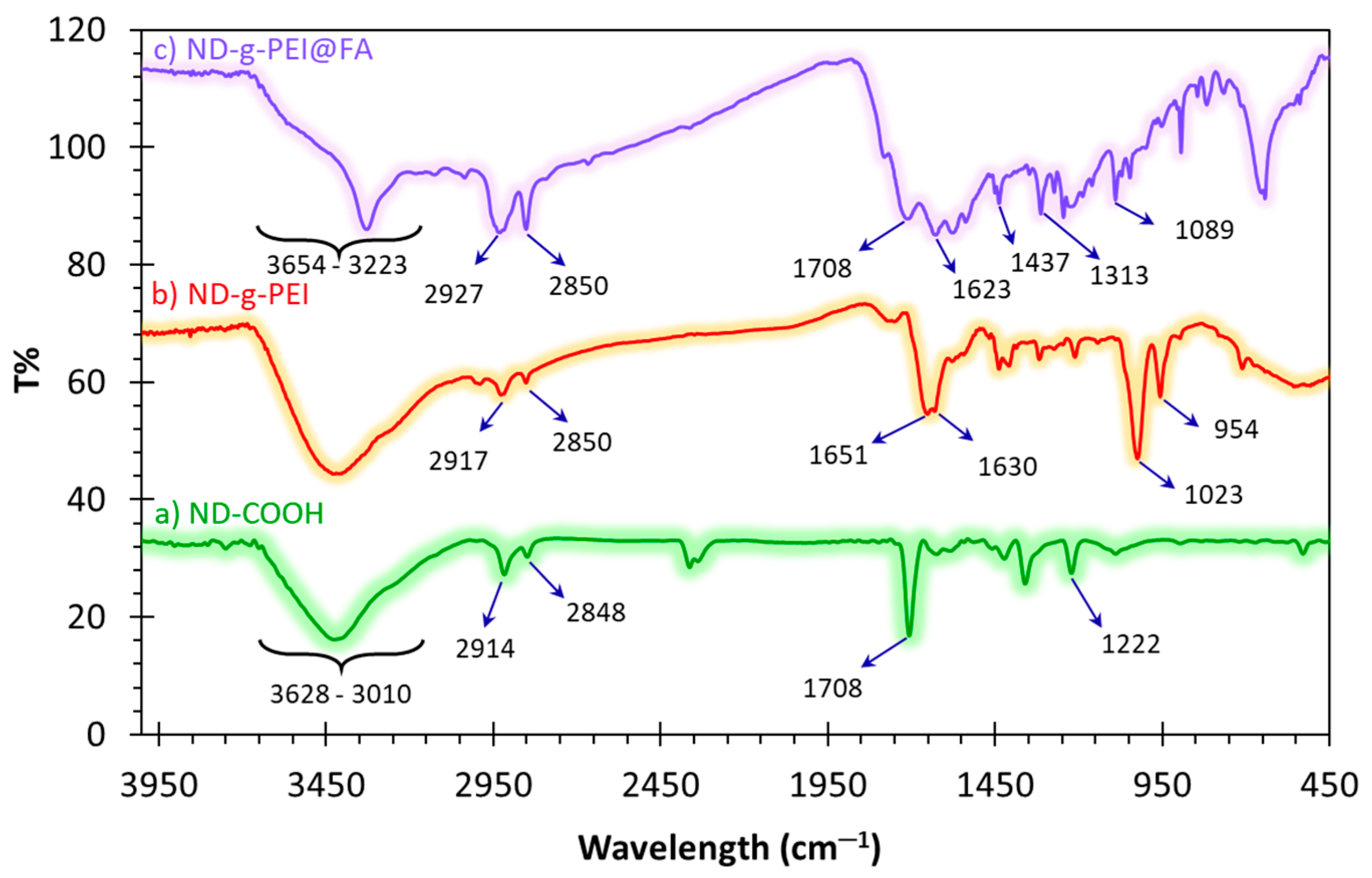
3.1. FT-IR Spectroscopy
3.2. EDS Analysis
3.3. XRD Pattern
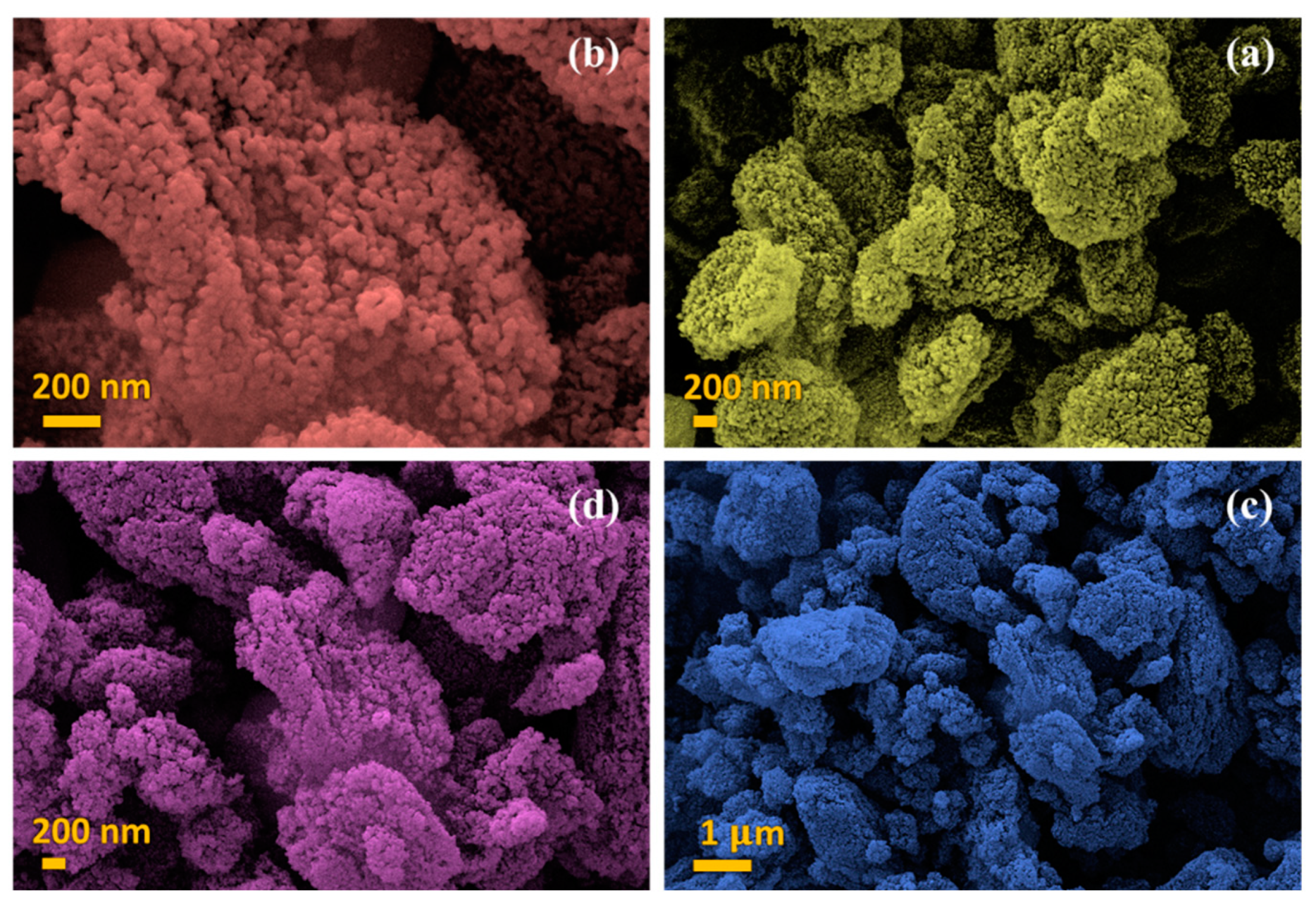
3.4. FE-SEM Analysis
3.5. Optimization of Reaction Conditions for the Synthesis of 5-Substituted 1H-Tetrazole Derivatives
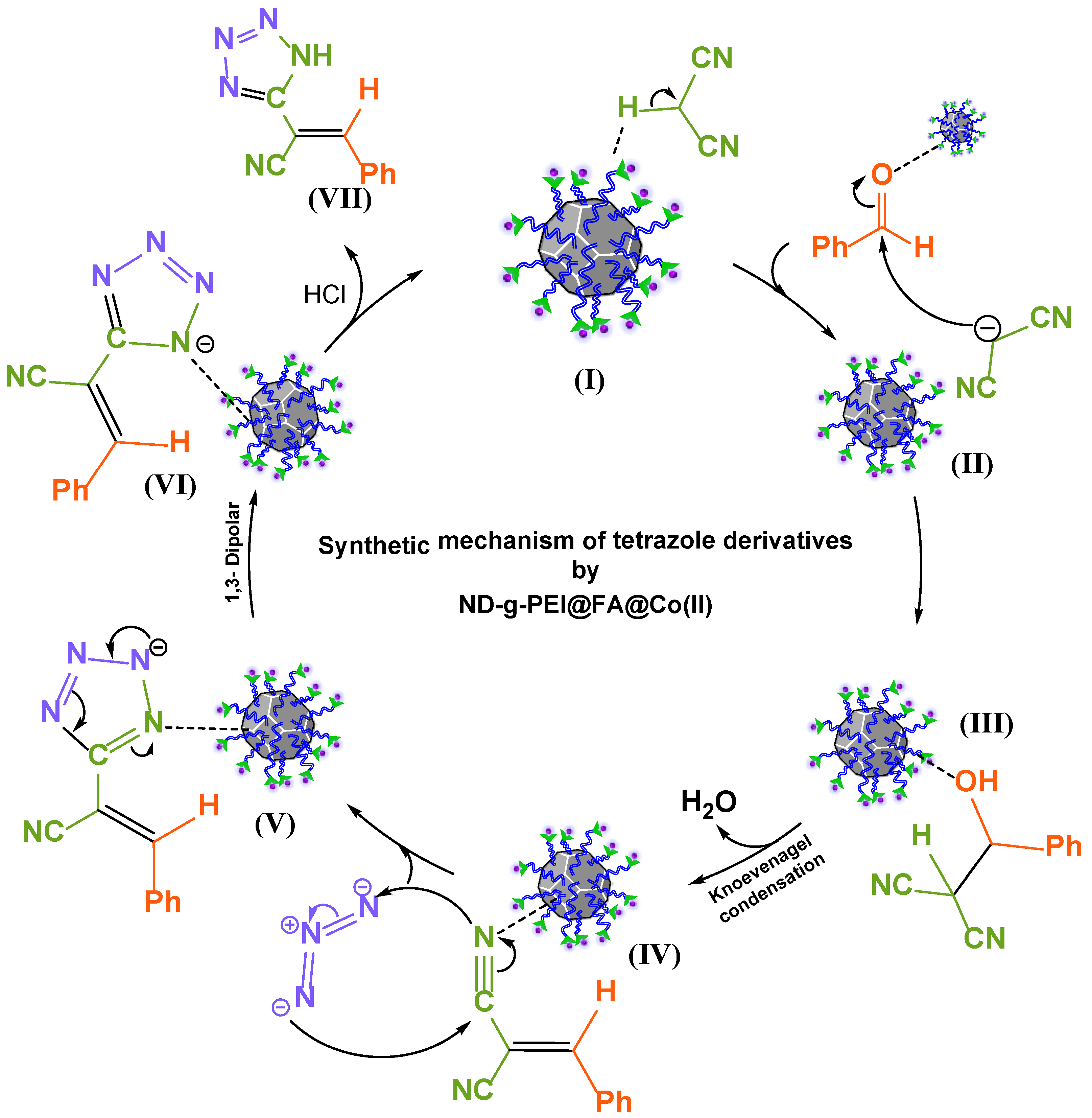
3.6. The Plausible Mechanism for the Synthesis of 5-Substituted 1H-Tetrazole Derivatives
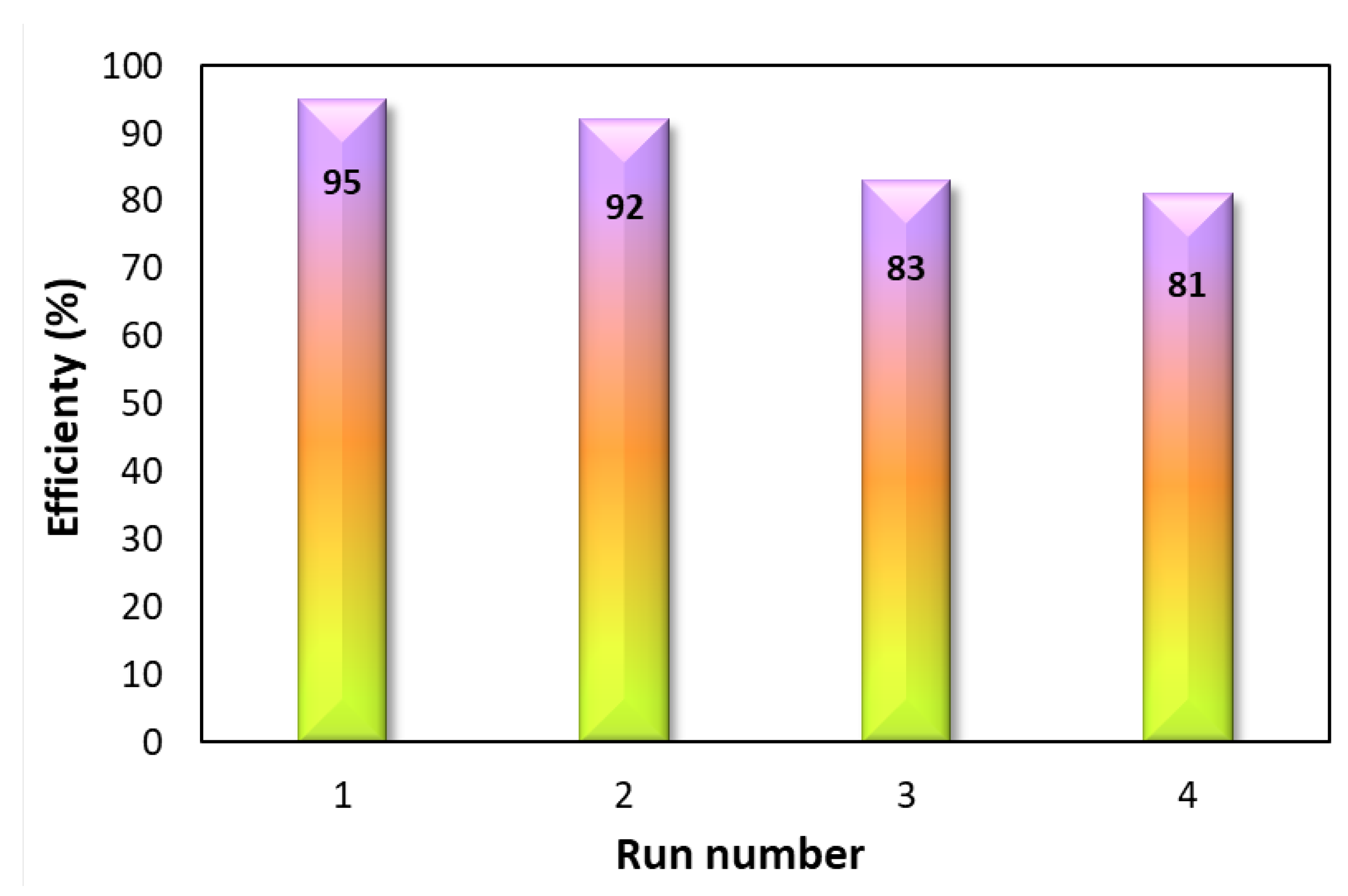
3.7. Reusability of ND-g-PEI@FA@Co(II) Nanocatalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maseer, M.M.; Kikhavani, T.; Tahmasbi, B. Multidentate copper complex on magnetic biochar nanoparticles as a practical and recoverable nanocatalyst for the selective synthesis of tetrazole derivatives. Nanoscale Adv. 2024, 6, 3948–3960. [Google Scholar] [CrossRef] [PubMed]
- Heidarnezhad, Z.; Ghorbani-Choghamarani, A.; Taherinia, Z. Magnetically recoverable Fe3O4@SiO2@SBA-3@2-ATP-Cu: An improved catalyst for the synthesis of 5-substituted 1 H-tetrazoles. Nanoscale Adv. 2024, 6, 4360–4368. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Łukowska-Chojnacka, E.; Mierzejewska, J.; Milner-Krawczyk, M.; Bondaryk, M.; Staniszewska, M. Synthesis of novel tetrazole derivatives and evaluation of their antifungal activity. Bioorganic Med. Chem. 2016, 24, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Biological Potentials of Substituted Tetrazole Compounds. Pharm. Methods 2014, 5, 39. [Google Scholar]
- Qian, A.; Zheng, Y.; Wang, R.; Wei, J.; Cui, Y.; Cao, X.; Yang, Y. Design, synthesis, and structure-activity relationship studies of novel tetrazole antifungal agents with potent activity, broad antifungal spectrum and high selectivity. Bioorganic Med. Chem. Lett. 2018, 28, 344–350. [Google Scholar] [CrossRef]
- Vembu, S.; Parasuraman, P.; Gopalakrishnan, M. Design, in silico molecular docking studies, synthesis, spectral characterization and in vitro antifungal evaluation of 1-(4-(1H-tetrazole-1-yl) phenyl)-3-arylprop-2-en-1-ones. Der Pharma Chem. 2014, 6, 35–44. [Google Scholar]
- DeMarinis, R.; Hoover, J.; Dunn, G.; Actor, P.; Uri, J.; Weisbach, J. A new parenteral cephalosporin, SK & F 59962: Chemistry and structure activity relationships. J. Antibiot. 1975, 28, 463–470. [Google Scholar]
- Vanam, N.R.; Anireddy, J.S. Synthesis and biological evaluation of tetrazole fused imidazopyridine derivatives as anticancer agents. Chem. Data Collect. 2023, 48, 101092. [Google Scholar] [CrossRef]
- Prabhu, D.J.; John, F.; Steephan, M.; John, J.; Sreehari, A.; Chandrika, B.B. Multicomponent reactions for the synthesis of tetrazole derivatives: Discovery and validation of a novel anticancer agent active against ER positive cancers. Results Chem. 2024, 7, 101470. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, V.; Mohite, P. Synthesis, characterization and evaluation of anticancer activity of some tetrazole derivatives. J. Optoelectron. Biomed. Mater. 2010, 2, 249–259. [Google Scholar]
- Chauhan, K.; Sharma, M.; Trivedi, P.; Chaturvedi, V.; Chauhan, P.M. New class of methyl tetrazole based hybrid of (Z)-5-benzylidene-2-(piperazin-1-yl) thiazol-4 (% H)-one as potent antitubercular agents. Bioorganic Med. Chem. Lett. 2014, 24, 4166–4170. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Li, Z.; Liu, X.; De Clercq, E. Sulfanyltriazole/tetrazoles: A promising class of HIV-1 NNRTIs. Mini Rev. Med. Chem. 2009, 9, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.A.; Jber, N.R.; Rasool, B.S.; Abbas, A.K. Tetrazole Derivatives and Role of Tetrazole in Medicinal Chemistry: An Article Review. Al-Nahrain J. Sci. 2023, 26, 1–7. [Google Scholar] [CrossRef]
- Babu, A.; Sinha, A. Catalytic Tetrazole Synthesis via [3+2] Cycloaddition of NaN3 to Organonitriles Promoted by Co (II)-complex: Isolation and Characterization of a Co (II)-diazido Intermediate. ACS Omega 2024, 9, 21626–21636. [Google Scholar] [CrossRef]
- Jabbari, A.; Moradi, P.; Tahmasbi, B. Synthesis of tetrazoles catalyzed by a new and recoverable nanocatalyst of cobalt on modified boehmite NPs with 1, 3-bis (pyridin-3-ylmethyl) thiourea. RSC Adv. 2023, 13, 8890–8900. [Google Scholar] [CrossRef]
- Norouzi, M.; Moradi, P.; Khanmoradi, M. Aluminium-based ionic liquid grafted on biochar as a heterogeneous catalyst for the selective synthesis of tetrazole and 2, 3-dihydroquinazolin 4 (1 H)-one derivatives. RSC Adv. 2023, 13, 35569–35582. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, R.; Zahedi, M. Magnetic Heterogeneous Catalyst Ni0.25Mn0.25Cu0.5Fe2O4: Its Efficiency in the Synthesis of Tetrazole Heterocycles with Biological Properties. Biol. Mol. Chem. 2024, 2, 1–12. [Google Scholar]
- Salimi, M.; Esmaeli-nasrabadi, F.; Sandaroos, R. Fe3O4@ Hydrotalcite-NH2-CoII NPs: A novel and extremely effective heterogeneous magnetic nanocatalyst for synthesis of the 1-substituted 1H-1, 2, 3, 4-tetrazoles. Inorg. Chem. Commun. 2020, 122, 108287. [Google Scholar] [CrossRef]
- Jabbari, A.; Moradi, P.; Hajjami, M.; Tahmasbi, B. Tetradentate copper complex supported on boehmite nanoparticles as an efficient and heterogeneous reusable nanocatalyst for the synthesis of diaryl ethers. Sci. Rep. 2022, 12, 11660. [Google Scholar] [CrossRef] [PubMed]
- Moradi, P.; Hajjami, M.; Tahmasbi, B. Fabricated copper catalyst on biochar nanoparticles for the synthesis of tetrazoles as antimicrobial agents. Polyhedron 2020, 175, 114169. [Google Scholar] [CrossRef]
- Molaei, S.; Ghadermazi, M. Ordered mesoporous MCM-41 containing cobalt ferrite as high-performance catalyst in the synthesis of 5-substituted 1H-tetrazoles and oxidation of sulfides. J. Porous Mater. 2024, 31, 1463–1476. [Google Scholar] [CrossRef]
- Swami, S.; Sahu, S.N.; Shrivastava, R. Nanomaterial catalyzed green synthesis of tetrazoles and its derivatives: A review on recent advancements. RSC Adv. 2021, 11, 39058–39086. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial effect of carbon nanomaterials: Nanotubes, carbon nanofibers, nanodiamonds, and onion-like carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef]
- Dias, L.D.; Rodrigues, F.M.; Calvete, M.J.; Carabineiro, S.A.; Scherer, M.D.; Caires, A.R.; Buijnsters, J.G.; Figueiredo, J.L.; Bagnato, V.S.; Pereira, M.M. Porphyrin–nanodiamond hybrid materials—Active, stable and reusable cyclohexene oxidation catalysts. Catalysts 2020, 10, 1402. [Google Scholar] [CrossRef]
- Ryu, T.K.; Baek, S.W.; Kang, R.H.; Choi, S.W. Selective photothermal tumor therapy using nanodiamond-based nanoclusters with folic acid. Adv. Funct. Mater. 2016, 26, 6428–6436. [Google Scholar] [CrossRef]
- Zhao, B.; Kolibaba, T.J.; Lazar, S.; Grunlan, J.C. Environmentally-benign, water-based covalent polymer network for flame retardant cotton. Cellulose 2021, 28, 5855–5866. [Google Scholar] [CrossRef]
- Luo, W.; Chi, R.; Zeng, F.; Wu, Y.; Chen, Y.; Liu, S.; Lin, W.; Lin, H.; Ye, X.; Chen, J. Multilayer structure ammoniated collagen fibers for fast adsorption of anionic dyes. ACS Omega 2021, 6, 27070–27079. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Salih, K.A.; Hamza, M.F.; Fujita, T.; Rodríguez-Castellón, E.; Guibal, E. Synthesis of a new phosphonate-based sorbent and characterization of its interactions with lanthanum (III) and terbium (III). Polymers 2021, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Su, C.; Cui, L.; Zhang, K.; Li, J. Preparing sodium alginate/polyethyleneimine spheres for potential application of killing tumor cells by reducing the concentration of copper ions in the lesions of colon cancer. Materials 2019, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, C.; Yan, Y.; Yang, L.; Liu, R.; Zhang, J.; Zhang, X.; Xie, C. Folic Acid Adjustive Polydopamine Organic Nanoparticles Based Fluorescent Probe for the Selective Detection of Mercury Ions. Polymers 2023, 15, 1892. [Google Scholar] [CrossRef] [PubMed]
- Bhanumathi, R.; Manivannan, M.; Thangaraj, R.; Kannan, S. Drug-carrying capacity and anticancer effect of the folic acid-and berberine-loaded silver nanomaterial to regulate the AKT-ERK pathway in breast cancer. ACS Omega 2018, 3, 8317–8328. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinezhad, A.; Akhlaghinia, B. Fe3O4@Boehmite-NH2-CoII NPs: An inexpensive and highly efficient heterogeneous magnetic nanocatalyst for the Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. Green Chem. 2017, 19, 5625–5641. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Facile synthesis of PEI-GO@ ZIF-8 hybrid material for CO2 capture. Int. J. Hydrog. Energy 2018, 43, 2224–2231. [Google Scholar] [CrossRef]
- Sumisha, A.; Arthanareeswaran, G.; Ismail, A.F.; Kumar, D.P.; Shankar, M.V. Functionalized titanate nanotube–polyetherimide nanocomposite membrane for improved salt rejection under low pressure nanofiltration. RSC Adv. 2015, 5, 39464–39473. [Google Scholar] [CrossRef]
- Akbarzadeh, P.; Koukabi, N.; Kolvari, E. Three-component solvent-free synthesis of 5-substituted-1 H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res. Chem. Intermed. 2019, 45, 1009–1024. [Google Scholar] [CrossRef]
- Tisseh, Z.N.; Dabiri, M.; Nobahar, M.; Khavasi, H.R.; Bazgir, A. Catalyst-free, aqueous and highly diastereoselective synthesis of new 5-substituted 1H-tetrazoles via a multi-component domino Knoevenagel condensation/1, 3 dipolar cycloaddition reaction. Tetrahedron 2012, 68, 1769–1773. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Paymard-Samani, S. Facile and rapid synthesis of 5-substituted 1 H-Tetrazoles VIA a multicomponent domino reaction using nickel (II) oxide nanoparticles as catalyst. Chem. Heterocycl. Compd. 2015, 50, 1567–1574. [Google Scholar] [CrossRef]
- Bakherad, M.; Doosti, R.; Keivanloo, A.; Gholizadeh, M.; Jadidi, K. Rapid, green, and catalyst-free one-pot three-component syntheses of 5-substituted 1H-tetrazoles in magnetized water. J. Iran. Chem. Soc. 2017, 14, 2591–2597. [Google Scholar] [CrossRef]
- Ara, M.; Ghafuri, H.; Ghanbari, N. Copper (II) anchored on layered double hydroxide functionalized guanidine as a heterogeneous catalyst for the synthesis of tetrazole derivatives. Colloid Interface Sci. Commun. 2023, 53, 100704. [Google Scholar] [CrossRef]



| Entry | Catalyst | Catalyst Dosage (mg) | Solvent | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | --- | --- | EtOH | r.t | 4 | N. R. a |
| 2 | --- | --- | Toluene | r.t | 4 | N. R. |
| 3 | --- | --- | DMF | r.t | 4 | N. R. |
| 4 | --- | --- | EtOH/H2O | r.t | 4 | N. R. |
| 5 | ND-COOH | 40 | EtOH/H2O | 100 | 4 | 60 |
| 6 | ND-g-PEI | 40 | EtOH/H2O | 100 | 4 | 72 |
| 7 | ND-g-PEI@FA | 40 | EtOH/H2O | 100 | 4 | 70 |
| 8 | ND-g-PEI@FA@Co(II) | 10 | EtOH/H2O | 80 | 4 | 80 |
| 9 | ND-g-PEI@FA@Co(II) | 20 | EtOH/H2O | 80 | 4 | 87 |
| 10 | ND-g-PEI@FA@Co(II) | 30 | EtOH/H2O | 80 | 4 | 89 |
| 11 | ND-g-PEI@FA@Co(II) | 40 | EtOH/H2O | 80 | 4 | 91 |
| 12 | ND-g-PEI@FA@Co(II) | 50 | EtOH/H2O | 80 | 4 | 90 |
| 13 | ND-g-PEI@FA@Co(II) | 40 | EtOH | 80 | 4 | 90 |
| 14 | ND-g-PEI@FA@Co(II) | 40 | Toluene | Reflux | 4 | 30 |
| 15 | ND-g-PEI@FA@Co(II) | 40 | DMF | Reflux | 4 | 50 |
| 16 | ND-g-PEI@FA@Co(II) | 40 | EtOH/H2O | 70 | 4 | 78 |
| 17 | ND-g-PEI@FA@Co(II) | 40 | EtOH/H2O | 90 | 4 | 95 |
| 18 | ND-g-PEI@FA@Co(II) | 40 | EtOH/H2O | 100 | 4 | 97 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Aldehyde (1a–m) | Product (4a–m) | Time (h) | Yield (%) | Mp (°C) | |
| Observed | Literature | |||||
| 1 | 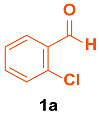 |  | 4 | 94 | 174–175 | 175–177 [33] |
| 2 | 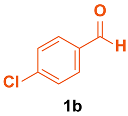 | 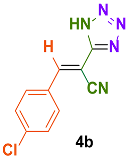 | 4 | 97 | 165–167 | 165–168 [33] |
| 3 | 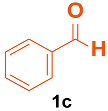 | 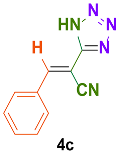 | 4 | 91 | 169–171 | 168–170 [34] |
| 4 | 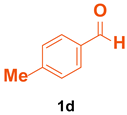 | 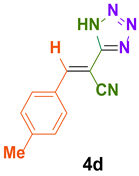 | 4 | 87 | 188–190 | 189–191 [35] |
| 5 | 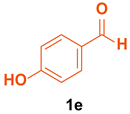 | 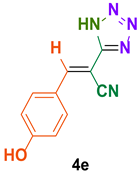 | 4 | 85 | 159–160 | 159–161 [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, Z.; Ramezani, A.; Ghafuri, H. Cobalt (II) Complex on Nanodiamond-Grafted Polyethyleneimine@Folic Acid: An Extremely Effective Nanocatalyst for Green Synthesis of 5-Substituted 1H-Tetrazole Derivatives. Chem. Proc. 2024, 16, 86. https://doi.org/10.3390/ecsoc-28-20132
Nasri Z, Ramezani A, Ghafuri H. Cobalt (II) Complex on Nanodiamond-Grafted Polyethyleneimine@Folic Acid: An Extremely Effective Nanocatalyst for Green Synthesis of 5-Substituted 1H-Tetrazole Derivatives. Chemistry Proceedings. 2024; 16(1):86. https://doi.org/10.3390/ecsoc-28-20132
Chicago/Turabian StyleNasri, Zahra, Arezoo Ramezani, and Hossein Ghafuri. 2024. "Cobalt (II) Complex on Nanodiamond-Grafted Polyethyleneimine@Folic Acid: An Extremely Effective Nanocatalyst for Green Synthesis of 5-Substituted 1H-Tetrazole Derivatives" Chemistry Proceedings 16, no. 1: 86. https://doi.org/10.3390/ecsoc-28-20132
APA StyleNasri, Z., Ramezani, A., & Ghafuri, H. (2024). Cobalt (II) Complex on Nanodiamond-Grafted Polyethyleneimine@Folic Acid: An Extremely Effective Nanocatalyst for Green Synthesis of 5-Substituted 1H-Tetrazole Derivatives. Chemistry Proceedings, 16(1), 86. https://doi.org/10.3390/ecsoc-28-20132







