Abstract
The synthesis and full characterization of new hydroxyl benzo[a]phenoxazinium chlorides with N-(di)propyl and/or N-isopropyl groups in the 5 and 9 positions are described. Photophysical studies carried out in ethanol and water revealed strong absorbances and significant fluorescence emissions at up to 676 nm. The antifungal activity was assessed against Saccharomyces cerevisiae and the results provide possible directions for further studies.
1. Introduction
Fungal infections represent a significant public health issue, especially due to the rising number of immunocompromised patients and the growing incidence of drug-resistant fungal strains. Although traditional antifungal treatments are effective, they often suffer from drawbacks such as toxicity, a narrow range of activity, and the potential for resistance. Consequently, there is a crucial need to discover new antifungal agents with innovative mechanisms of action [1,2,3,4]. In this regard, benzo[a]phenoxazines have surfaced as promising candidates [5,6,7,8,9].
Benzo[a]phenoxazines are a class of heterocyclic compounds known for their diverse biological activities, including their antimicrobial, anticancer, and antiviral properties. Their unique structure, characterized by a fused aromatic system, imparts distinctive electronic and photophysical properties, making them attractive for various biomedical applications [10,11,12,13,14,15,16,17,18,19,20].
Given our research team’s interest in this type of compound [5,6,7,8,9,11,12,13,14,15,16,17,18,19,20], the present work is focused on the synthesis of three hydroxyl benzo[a]phenoxazinium chlorides di- or mono-substituted with (iso)propyl groups at the amines of the 5 and 9 positions of the polycyclic system. A photophysical characterization of the synthesized compounds was carried out and their potential antifungal activity was evaluated.
2. Results and Discussion
The precursors of the target benzo[a]phenoxazine derivatives, namely the nitrosophenol hydrochlorides, were prepared via the nitrosation of 3-(dipropylamino)phenol or 3-(propylamino)phenol with sodium nitrite in the presence of hydrochloric acid. In addition, 5-aminonaphthalen-2-ol was N-alkylated with 1-bromopropane or 2-bromopropane in ethanol to obtain the other required precursor.
The condensation of 5-(dipropylamino)-2-nitrosophenol or 2-nitroso-5-(propylamino)phenol with 5-(propylamino)naphthalen-2-ol or 5-(isopropylamino)naphthalen-2-ol, with hydrochloric acid, in ethanol, yielded the corresponding hydroxyl benzo[a]phenoxazinium chlorides 1a–c as blue solids, with yields of up to 39% (Scheme 1). The full characterization of these compounds was carried out via the usual analytical techniques.
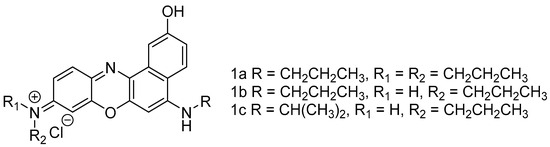
Scheme 1.
Structures of hydroxyl benzo[a]phenoxazinium chlorides 1a–c.
The 1H NMR spectra of compounds 1a–c showed the methyl groups as triplets, multiplets, or a doublet (1c) (δ 1.06–1.50 ppm), the adjacent methylene protons as sextets or multiplets (δ 1.71–1.93 ppm), methylene protons adjacent to the nitrogen atoms as triplets or multiplets (δ 3.30–3.70 ppm), and the proton of tertiary carbon as a quintet (1c), in addition to the aromatic protons (δ 6.78–8.33 ppm). In the case of 13C NMR spectra, it was possible to confirm the presence of the methyl (δ 11.20–22.08 ppm) and methylene (δ 46.28–54.46 ppm) carbons of (iso)propyl groups, as well as the aromatic carbons of the benzo[a]phenoxazinium core (δ 93.06–162.97 ppm).
The photophysical study of the benzo[a]phenoxazinium chlorides 1a–c was based on the absorption and emission spectra of 10−6 M solutions in ethanol and water. Oxazine 1 was used as the standard (ΦF = 0.11, in ethanol), with excitation at 590 nm, for the determination of the relative fluorescence quantum yields (ΦF). Table 1 shows a summary of the results.

Table 1.
Photophysical data of compounds 1a–c in ethanol and water (λexc 590 nm).
In ethanol and water, maximum absorption wavelengths (λabs) for all compounds were found in the range 612–634 nm, with molar extinction coefficients (ɛ) between 17,460 and 44,613 M−1cm−1. The maximum emission wavelengths (λemi) lie in the range of 644–676 nm at an excitation of 590 nm, with moderate Stokes’ shifts (∆λ, 23–42 nm).
In ethanol, the λabs values are very similar for the three compounds, although they were higher for 1a. On the other hand, in water, there was a bathochromic shift in 1a of 18 and 22 nm compared to 1b and 1c, respectively. This occurs due to the di-alkylation of the amine in the 9 position, as noted earlier [14]. The decrease in ɛ values observed in all compounds when switching from ethanol to water may be related to differences in the solubility of the compounds in the two solvents.
Regarding the λemi, in ethanol, it is practically the same for all three compounds. Nevertheless, in water, as occurred for the λabs values, compound 1a underwent a bathochromic shift of 26 and 25 nm in relation to 1b and 1c.
The fluorescence quantum yields (ΦF) were in the range 0.10 to 0.59, with the best values obtained for all compounds in ethanol (0.54–0.59). The decrease in ΦF in water, although present in all three compounds, was more significant in the case of 1a (0.10) than in 1b (0.28) and 1c (0.27). However, the values obtained in water continued to be very interesting given the limited number of fluorophores with emissions at high wavelengths that showed considerable solubility in aqueous media.
The normalized absorption and emission spectra of the benzo[a]phenoxazinium chlorides 1a–c in ethanol and water are shown in Figure 1 and Figure 2, respectively.
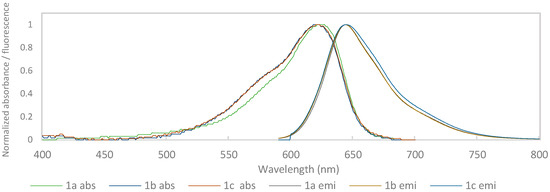
Figure 1.
Normalized absorbance and emission spectra of compounds 1a–c in ethanol.
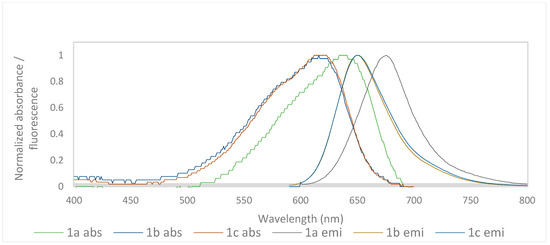
Figure 2.
Normalized absorbance and emission spectra of compounds 1a–c in water.
Benzo[a]phenoxazinium chlorides 1a–c were evaluated to determine their antifungal activity against Saccharomyces cereviseae PYCC 4072. The minimum concentration of each compound at which the yeast growth was inhibited by ≥80%, denoted as the Minimum Inhibitory Concentration (MIC) value, was superior to 200 µM for all the compounds. These results suggest that the hydroxyl group in position 2 of the polycyclic system decreases the potential antifungal activity of benzo[a]phenoxazines, since the presence of (di)propyl groups in the amines at positions 5 and 9 of the polycyclic system when this hydroxyl group is not present is usually associated with low MIC values [7,13]. This fact stands out in the non-hydroxylated benzo[a]phenoxazinium chloride analogous to compound 1a, with an MIC value of 1.56 [7]. However, the effect of the hydroxyl group on the antifungal activity of benzophenoxazine derivatives needs to be further evaluated with a higher number of compounds to draw conclusions about the structure–activity relationship.
3. Experimental
3.1. Typical Procedure for the Preparation of Compounds 1a–c (Illustrated for 1a)
Concentrated hydrochloric acid (5 × 10−2 mL) was added to a solution of 5-(dipropylamino)-2-nitrosophenol hydrochloride (0.125 g, 5.69 × 10−4 mol) in ethanol (2 mL), followed by 5-(propylamino)naphthalen-2-ol (0.057 g, 2.84 × 10−4 mol), and the resulting solution was refluxed for 15 h. The reaction progress was monitored via TLC (dichloromethane/methanol 98:2). After evaporation of the solvent and column chromatography purification on silica gel (mixtures with an increase in the polarity of dichloromethane/methanol as the eluent), N-(2-hydroxy-5-(propylamino)-9H-benzo[a]phenoxazin-9-ylidene)-N-propylpropan-1-aminium chloride 1a was obtained as a blue solid (0.049 g, 39%). υmáx (solid) 3400, 3200, 2872, 1640, 1592, 1557, 1457, 1428, 1332, 1168, 1125, 1099, 1034, 917, 822, 754 cm−1. δH (CD3OD, 400 MHz) 1.06 (t, J = 7.2 Hz, 6H, N(CH2CH2CH3)2), 1.12 (t, J = 7.2 Hz, 3H, NHCH2CH2CH3), 1.71–1.84 (m, 4H, N(CH2CH2CH3)2), 1.87–1.93 (m, 2H, NHCH2CH2CH3), 3.60 (t, J = 8 Hz, 4H, N(CH2CH2CH3)2), 3.70 (t, J = 7.2 Hz, 2H, NHCH2CH2CH3), 6.87 (broad s, 2H, H-6 and H-8), 7.20–7.30 (m, 2H, H-10 and H-11), 7.84 (d, J = 9.2 Hz, 1H, H-3), 8.23 (broad s, 2H, H-4 and H-1) ppm. δC (CD3OD, 100.6 MHz) 11.20 (N(CH2CH2CH3)2 or NHCH2CH2CH3), 11.46 (N(CH2CH2CH3)2 or NHCH2CH2CH3), 11.74 (N(CH2CH2CH3)2 or NHCH2CH2CH3), 20.69 (N(CH2CH2CH3)2), 21.76 (N(CH2CH2CH3)2), 23.26 (NHCH2CH2CH3), 50.48 (NHCH2CH2CH3), 54.46 (N(CH2CH2CH3)2), 93.51 (C-8), 97.13 (C-6), 110.09 (C-1), 115.80 (C-10), 117.05 (Ar-C), 120.14 (C-11), 126.35 (Ar-C), 130.35 (C-4), 133.67 (Ar-C), 135.22 (C-3), 135.60 (Ar-C), 149.30 (Ar-C), 153.41 (Ar-C), 155.56 (C-9), 159.65 (C-5) 162.97 (C-2) ppm.
3.2. Procedure for Antifungal Activity Tests
The Minimum Inhibitory Concentration of growth for benzo[a]phenoxazinium chlorides 1a–c was determined through the broth microdilution method to test the antifungal susceptibility of yeasts (M27-A3, CLSI—Clinical and Laboratory Standards Institute). The incubation of cells was carried out at 30 °C in RPMI 1640 medium buffered to pH 7.0 with a 0.165 M morpholenepropanesulfonic acid (MOPS) buffer. The initial cell concentration was 2.25 × 103 cells/mL. Stock solutions of the benzo[a]phenoxazinium chlorides 1a–c were prepared in DMSO and a final dilution was performed in an RPMI 1640 medium (DMSO concentrations of 0.5% per well). After 48 h of incubation, the optical density of the cultures was read using a microplate photometer. MIC values were considered to be the lowest concentration of the drug that resulted in a growth inhibition over 80% when compared to the growth control containing 0.5% DMSO. All the drug concentrations were tested in triplicate and in two independent experiments.
4. Conclusions
New hydroxyl benzo[a]phenoxazinium chlorides were successfully synthetized and spectroscopically characterized. Studies of their photophysical properties in ethanol and water revealed that these compounds showed fluorescence with λemi in the range of 644 to 683 nm, and fluorescent quantum yields of up to 0.59. The highest values were found in ethanol for compound 1a, with the N-dipropyl group in the 9 position, and in water for compound 1b, with only one propyl group found in the nine-amine position. In terms of activity, it was found that the presence of the hydroxyl group in position 2 of the polycyclic system reduces biological activity compared to non-hydroxylated analogues. However, the low toxicity of the new benzo[a]phenoxazinium chlorides could be beneficial to their use as fluorescent markers in biological applications given their interesting photophysical properties and solubility in aqueous media.
Author Contributions
Conceptualization, M.S.T.G. and M.J.S.; methodology, M.S.T.G. and M.J.S.; investigation, B.S.G.G.; writing—original draft preparation, M.S.T.G.; writing—review and editing, M.S.T.G. and M.J.S.; supervision, M.S.T.G. and M.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research centres CQ-UM (UID/QUI/00686/2020) and CBMA (UIDB/04050/2020) were funded by the Foundation for Science and Technology (FCT, Portugal), and FEDER-COMPETE-QREN-EU. The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network (PTNMR) and is partially supported by Infrastructure Project No. 022161 (co-financed by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal drug resistance: An emergent health threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.G.; Diekema, D.J. What is new in fungal infections? Mod. Pathol. 2023, 36, 100187. [Google Scholar] [CrossRef]
- Yiu, B.; Robbins, N.; Cowen, L.E. Interdisciplinary approaches for the discovery of novel antifungals. Trends Mol. Med. 2024, 30, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Reguera-Gomez, M.; Dores, M.R.; Martinez, L.R. Innovative and potential treatments for fungal central nervous system infections. Curr. Opin. Microbiol. 2023, 76, 102397. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Sousa, M.J.; Moura, J.C.V.P.; Gonçalves, M.S.T. Synthesis of naphtho[2,3-a]phenoxazinium chlorides. Structure-activity relationships of these heterocycles and benzo[a]phenoxazinium chlorides as new antimicrobials. Bioorg. Med. Chem. 2008, 16, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Alves, C.T.; Raju, B.R.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B Biol. 2014, 141, 93–99. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Raju, B.R.; Naik, S.; Coutinho, P.J.G.; Sousa, M.J.; Gonçalves, M.S.T. Synthesis and photophysical studies of new benzo[a]phenoxazinium chlorides as potential antifungal agents. Tetrahedron Lett. 2016, 57, 3936–3941. [Google Scholar] [CrossRef]
- Raju, B.R.; Leitão, M.I.P.S.; Sousa, M.J.; Coutinho, P.J.G.; Gonçalves, M.S.T. New NIR dyes based on quinolizino[1,9-hi]phenoxazin-6-iminium chlorides: Synthesis, photophysics and antifungal activity. Dyes Pigment. 2020, 173, 107870. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Raju, B.R.; Cerqueira, N.M.F.S.A.; Sousa, M.J.; Gonçalves, M.S.T. Benzo[a]phenoxazinium chlorides: Synthesis, antiproliferative activity, in silico studies and evaluation as fluorescent probes. Bioorg. Chem. 2020, 98, 103730. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and nile blue: Applications and synthesis of structural analogues. Chem. Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Ferreira, J.C.C.; Lopes, C.; Preto, A.; Gonçalves, M.S.T.; Sousa, M.J. Novel nile blue analogue stains yeast vacuolar membrane, endoplasmic reticulum and lipid droplets, inducing cell death through vacuole membrane permeabilization. J. Fungi 2021, 7, 971. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Granja, S.; Almeida, A.F.; Baltazar, F.; Gonçalves, M.S.T.; Preto, A.; Sousa, M.J. Targeting lysosomes in colorectal cancer: Exploring the anticancer activity of a new benzo[a]phenoxazine derivative. Int. J. Mol. Sci. 2023, 24, 614. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Sousa, R.P.C.L.; Preto, A.; Sousa, M.J.; Gonçalves, M.S.T. Novel benzo[a]phenoxazinium chlorides functionalized with sulfonamide groups as NIR fluorescent probes for vacuole, endoplasmic reticulum, and plasma membrane staining. Int. J. Mol. Sci. 2023, 24, 3006. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.R.; Carvalho, M.M.T.; Leitão, M.I.P.S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis, photophysical characterisation and photostability studies of NIR probes with aliphatic, aromatic and chlorinated terminals in 5- and 9-amino positions of benzo[a]phenoxazines. Dyes Pigment. 2016, 132, 204–212. [Google Scholar] [CrossRef][Green Version]
- Raju, B.R.; Gonçalves, M.S.T.; Coutinho, P.J.G. Fluorescent probes based on side-chain chlorinated benzo[a]phenoxazinium chlorides: Studies of interaction with DNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel DNA fluorescence probes based on N-[5-(11-functionalised-undecylamino)-9H-benzo[a]phenoxazin-9-ylidene]propan-1-aminium chlorides: Synthesis and photophysical studies. Tetrahedron Lett. 2011, 52, 112–116. [Google Scholar] [CrossRef]
- Naik, S.; Alves, C.M.A.; Coutinho, P.J.G.; Gonçalves, M.S.T. N-(Di)icosyl-substituted benzo[a]phenoxazinium chlorides: Synthesis and evaluation as near-infrared membrane probes. EurJOC 2011, 2011, 2491–2497. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Coutinho, P.J.G.; Moura, J.C.V.P.; Gonçalves, M.S.T. Functionalised benzo[a]phenoxazine dyes as long-wavelength fluorescent probes for amino acids. Tetrahedron 2007, 63, 1654–1663. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Gonçalves, M.S.T. Fluorescence derivatisation of amino acids in short and long-wavelengths. Tetrahedron Lett. 2007, 48, 3403–3407. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of short and long-wavelength functionalised probes: Amino acids’ labelling and photophysical studies. Tetrahedron 2007, 63, 12405–12418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).