Evaluation of the Photostability of Ivermectin †
Abstract
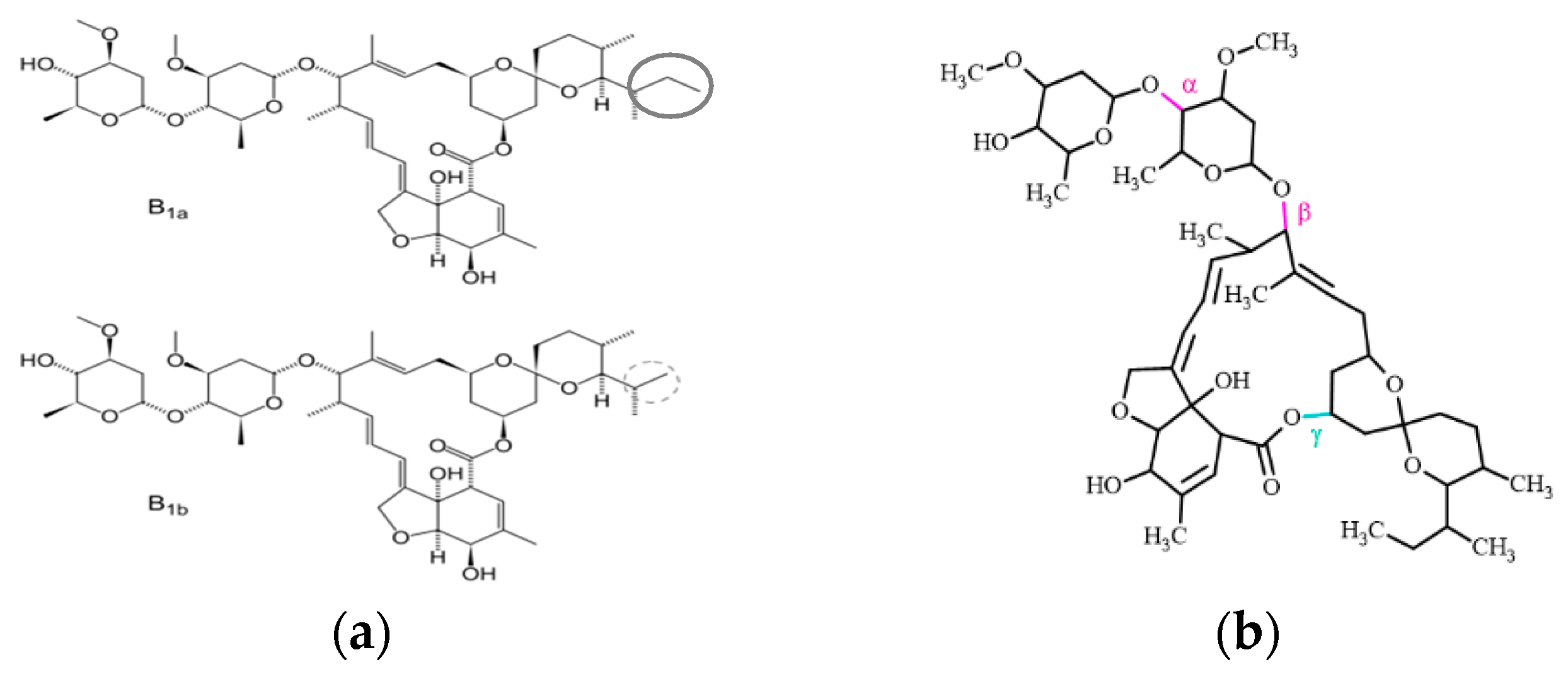
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Methods
2.2.1. Sample Preparation
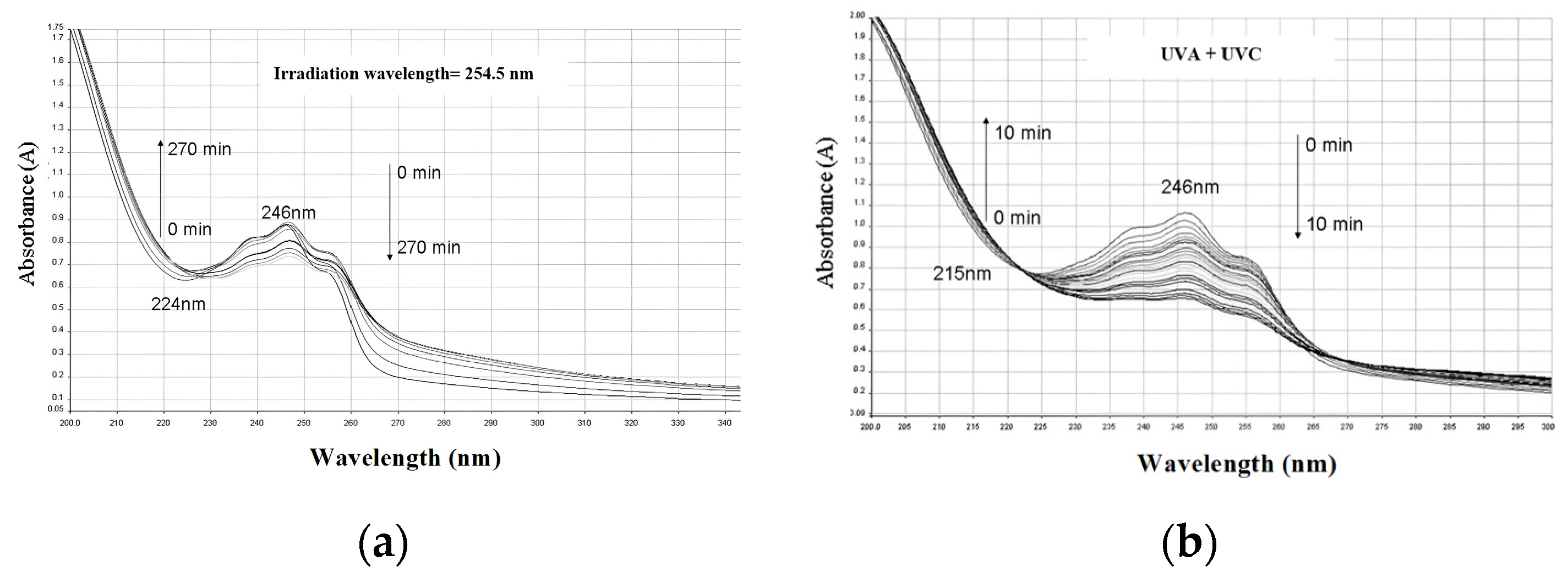
2.2.2. Evaluation of Photostability by UV-Visible Spectrometry
2.2.3. Phototoxicity on Human Erythrocytes
2.2.4. Computational Calculations
3. Discussion and Results
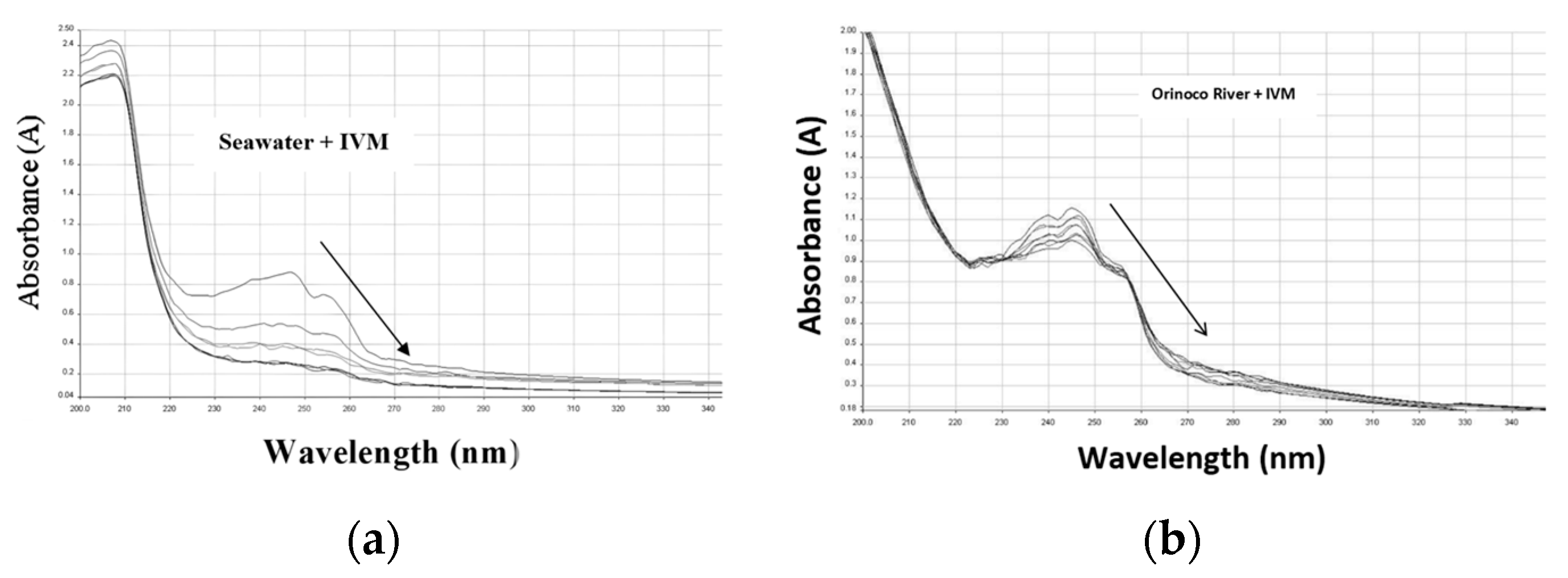
3.1. Photostability of IVM
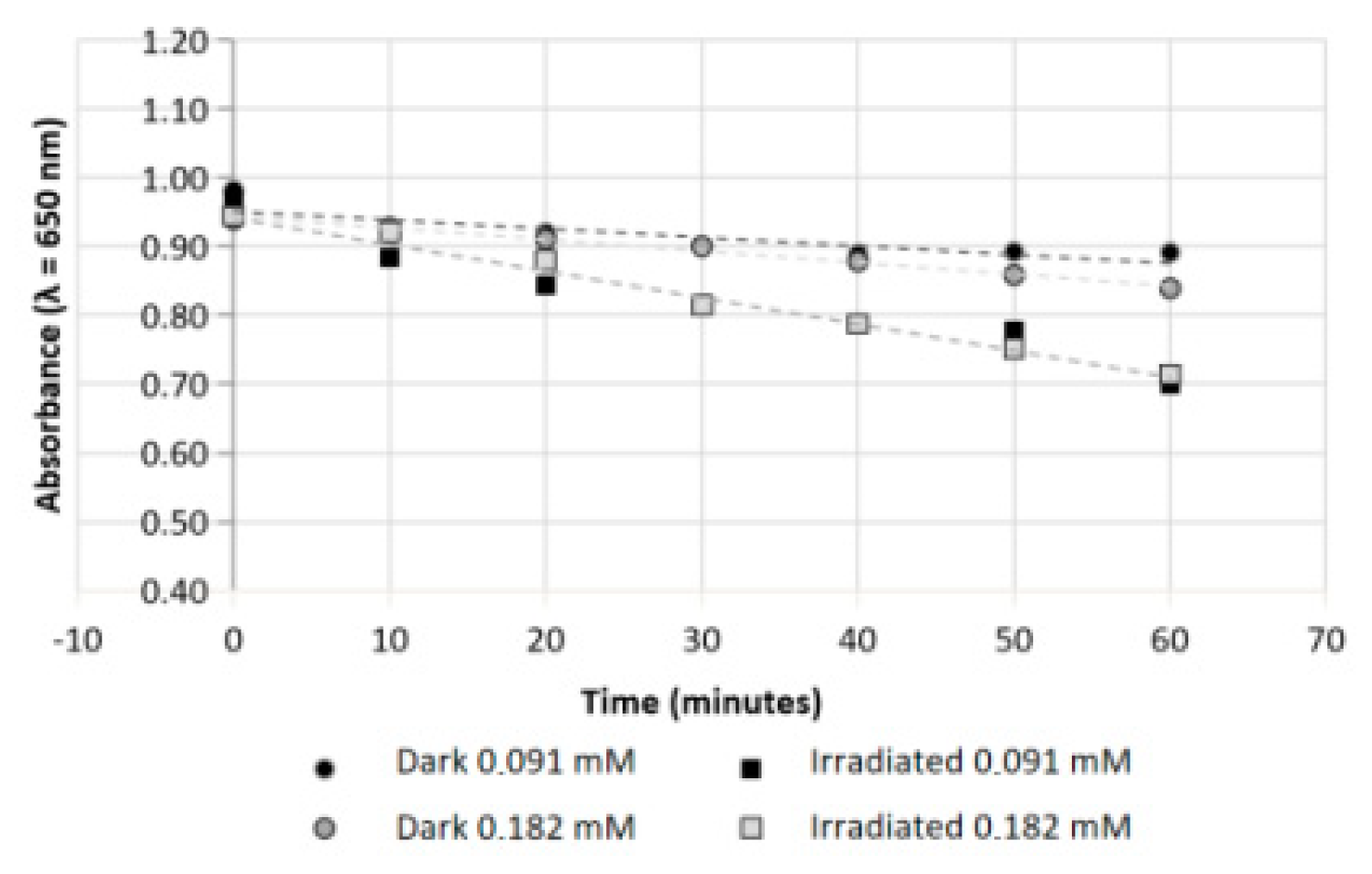
3.2. Photohemolytic Effects of IVM
3.3. Computational Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crump, A.; Ōmura, S. Ivermectin, ‘wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 87, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Geary, T. Ivermectin 20 years on: Maturation of a wonder drug. Trends Parasitol. 2005, 21, 530–532. [Google Scholar] [CrossRef] [PubMed]
- González, P.; González, F.A.; Ueno, K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr. Pharm. Biotechnol. 2012, 13, 1103–1109. [Google Scholar] [CrossRef]
- Shirazi, F.; Mirzaei, R.; Nakhaee, S.; Nejatian, A.; Ghafari, S.; Mehrpour, O. Repurposing the drug, ivermectin, in COVID-19: Toxicological points of view. Eur. J. Med. Res. 2022, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Sahagún, P.; Diez, L.; Fernández, N.; Sierra, M.; García, J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Õmura, S.; Crump, A. The life and times of ivermectin—A success story. Nat. Rev. Microbiol. 2004, 2, 984–989. [Google Scholar] [CrossRef]
- Roz, L.; Victoria, G.; Eileen, D. Ivermectin–Old Drug, New Tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef]
- David, W. Fink, Ivermectin. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1988; Volume 17, pp. 155–184. [Google Scholar] [CrossRef]
- Mirta, Č.; Davor, L.; Lidija, Ć.; Irena, Š.; Sandra, B. Kinetics and degradation pathways of photolytic and photocatalytic oxidation of the anthelmintic drug praziquantel. J. Hazard. Mater. 2017, 323, 500–512. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chern. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Selvasembian, R.; Offiong, N.A.O.; El Din Mahmoud, A.; Sanganyado, E.; Mal, J. COVID-19 drugs in aquatic systems: A review. Environ. Chem. Lett. 2022, 20, 1275–1294. [Google Scholar] [CrossRef]
- John, R.E.; John, I.H.; Allan, H.D.; Jefrey, E.R. Dissolved humic substances of the Amazon River system. Limnol. Oceanogr. 1986, 31, 139–154. [Google Scholar] [CrossRef]
- Peiris, R.; Budman, H.; Moresoli, C.; Legge, R. Identification of humic acid-like and fulvic acid-like natural organic matter in river water using fluorescence spectroscopy. Water Sci. Technol. 2011, 63, 2427–2433. [Google Scholar] [CrossRef]
- Kahn, G.; Fleischaker, B.I. Red Blood Cell Hemolysis by Photosensitizing Compounds. J. Investig. Dermatol. 1971, 56, 85–90. [Google Scholar] [CrossRef][Green Version]
- Costanzo, L.; De Guidi, G.; Condorelli, G.; Cambria, A.; Fama, M. Molecular mechanism of drug photosensitization–II. Photohemolysis sensitized by ketoprofen. Photochem. Photobiol. 1989, 50, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Havlíkova, L.; Satínský, D.; Solich, P. Aspects of decontamination of ivermectin and praziquantel from environmental waters using advanced oxidation technology. Chemosphere 2016, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, F.; León, M.; Angulo, B.; Álvarez, Á.; González, J.; Maldonado, A. Evaluation of the Photostability of Ivermectin. Chem. Proc. 2024, 16, 78. https://doi.org/10.3390/ecsoc-28-20182
Vargas F, León M, Angulo B, Álvarez Á, González J, Maldonado A. Evaluation of the Photostability of Ivermectin. Chemistry Proceedings. 2024; 16(1):78. https://doi.org/10.3390/ecsoc-28-20182
Chicago/Turabian StyleVargas, Franklin, Miguel León, Beatriz Angulo, Álvaro Álvarez, Jhonatan González, and Alexis Maldonado. 2024. "Evaluation of the Photostability of Ivermectin" Chemistry Proceedings 16, no. 1: 78. https://doi.org/10.3390/ecsoc-28-20182
APA StyleVargas, F., León, M., Angulo, B., Álvarez, Á., González, J., & Maldonado, A. (2024). Evaluation of the Photostability of Ivermectin. Chemistry Proceedings, 16(1), 78. https://doi.org/10.3390/ecsoc-28-20182





