Design, Synthesis, and Structural Study of a Shiff Base Ligand Precursor of Metallosupramolecular Architectures †
Abstract
1. Introduction
2. Experimental Section
2.1. Reactants and Solvents
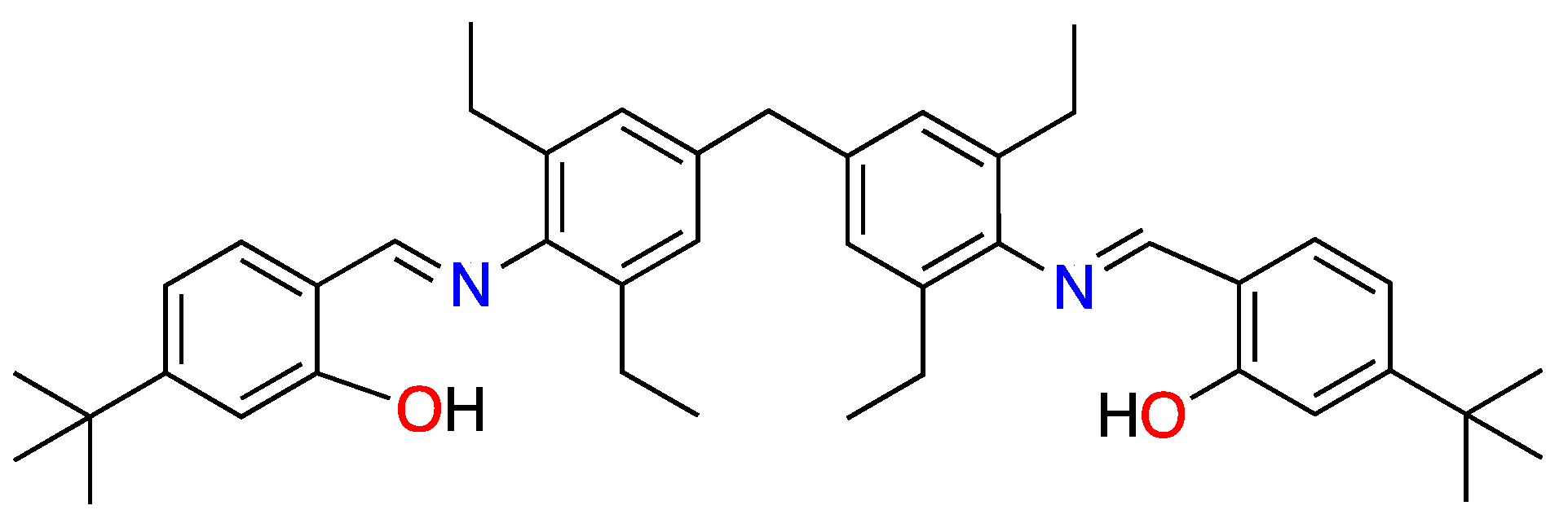
2.2. Synthesis and Characterization of the Schiff Base Ligand H2L
2.3. Crystallographic Data of H2L
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taha, R.H.; El-Shafiey, Z.A.; Salman, A.A.; El-Fakharany, E.M.; Mansour, M.M. Synthesis and characterization of newly synthesized Schiff base ligand and its metal complexes as potent anticancer. J. Mol. Struct. 2019, 1181, 536–545. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs. Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Sahu, R.; Shah, K. Schiff Bases: A Captivating Scaffold with Potential Anticonvulsant Activity. Mini-Rev. Med. Chem. 2024, 24, 1632–1650. [Google Scholar] [CrossRef] [PubMed]
- Malav, R.; Ray, S. Recent developments on the synthesis of copper and cobalt-Schiff base complexes and their assessment as anti-tuberculosis drugs. Chem. Pap. 2024, 78, 4623–4646. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Jorge, J.; Del Pino Santos, K.F.; Timóteo, F.; Piva Vasconcelos, R.R.; Ignacio Ayala Cáceres, O.; Juliane Arantes Granja, I.; de Souza, D.M.; Frizon, T.E.A.; Di Vaccari Botteselle, G.; Braga, A.L.; et al. Recent Advances on the Antimicrobial Activities of Schiff Bases and their Metal Complexes: An Updated Overview. Curr. Med. Chem. 2024, 31, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff bases and their metal Complexes: A review on the history, synthesis, and applications. Inorg. Chem. Commun. 2023, 150, 110451. [Google Scholar] [CrossRef]
- Ding, X.; Wang, L.; Chang, Y.; Wei, C.; Lin, J.; Ding, M.; Huang, W. Schiff base flexible organic crystals toward multifunctional applications. Aggregate 2024, 5, e500. [Google Scholar] [CrossRef]
- Mezgebe, K.; Mulugeta, E. Synthesis and pharmacological activities of Schiff bases with some transition metal complexes: A review. Med. Chem. Res. 2024, 33, 439–463. [Google Scholar] [CrossRef]
- Pramanik, K.; Jana, N.C.; Sun, Y.C.; Brandäo, P.; Wang, X.Y.; Saha, A.; Panja, A. Structure and magnetism of DyIII2 based triple-stranded helicates derived from dinucleating Schiff base ligands. Inorg. Chim. Acta 2024, 571, 122219. [Google Scholar] [CrossRef]
- Konishi, Y.; Ehara, T.; Cui, L.; Ueno, K.; Ishigaki, Y.; Harada, T.; Konta, T.; Onda, K.; Hoshino, Y.; Miyata, K.; et al. Optical Property Control by the Interligand Charge Transfer Excited State in Brominated Homoleptic and Heteroleptic Aluminum Dinuclear Triple-Stranded Helicates. Inorg. Chem. 2024, 63, 11716–11725. [Google Scholar] [CrossRef]
- Fernández-Fariña, S.; Velo-Heleno, I.; Martínez-Calvo, M.; Maneiro, M.; Pedrido, R.; González-Noya, A.M. Schiff Bases Functionalized with T-Butyl Groups as Adequate Ligands to Extended Assembly of Cu(II) Helicates. Int. J. Mol. Sci. 2023, 24, 8654. [Google Scholar] [CrossRef] [PubMed]
- Andruh, M. The exceptionally rich coordination chemistry generated by Schiff-base ligands derived from o-vanillin. Dalton Trans. 2015, 44, 16633–16653. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fariña, S.; Maneiro, M.; Zaragoza, G.; Seco, J.M.; Pedrido, R.; González-Noya, A.M. Nickel, copper, and zinc dinuclear helicates: How do bulky groups influence their architecture? Dalton Trans. 2024, 53, 5676–5685. [Google Scholar] [CrossRef] [PubMed]
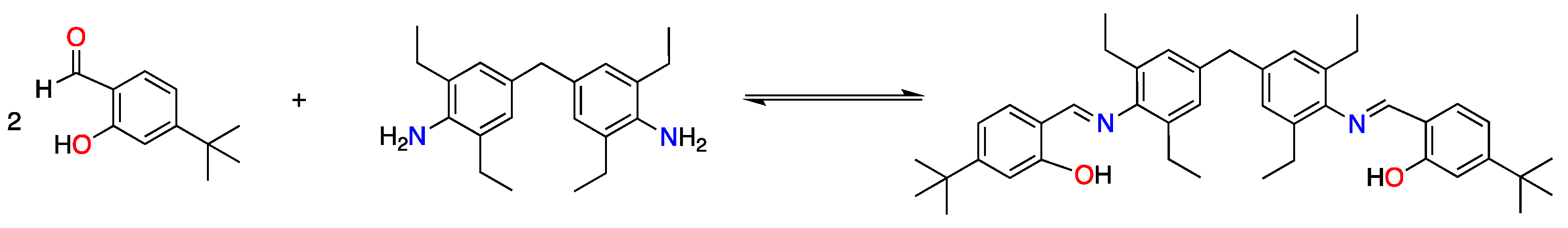
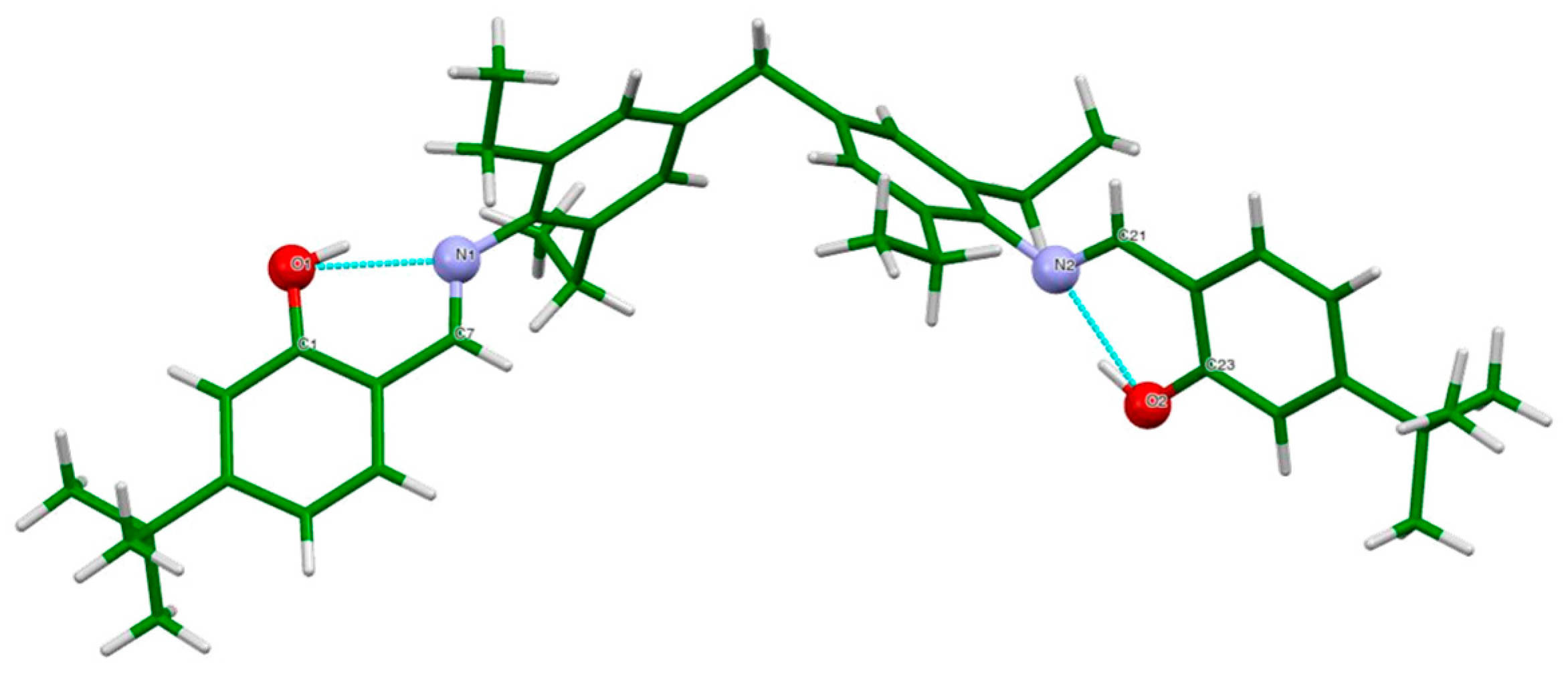
| Main Bond Distances (Å) | |||
|---|---|---|---|
| 01–C1 | 1.3502 (16) | O2–C23 | 1.3550 (17) |
| N1–C7 | 1.2791 (17) | N2–C21 | 1.279 (2) |
| N1–C8 | 1.4292 (17) | N2–C18 | 1.4377 (19) |
| C1–C6 | 1.4045 (19) | C21–C22 | 1.456 (2) |
| C6–C7 | 1.4513 (19) | C22–C23 | 1.403 (2) |
| Angles (°) | |||
| C1–N1–C8 | 119.69 (11) | C17–C18–N2 | 199.99 (14) |
| N1–C7–C6 | 122.37 (12) | C21–N2–C18 | 117.59 (15) |
| C9–C8–N1 | 117.38 (12) | N2–C18–C19 | 116.97 (15) |
| C13–C8–N1 | 121.13 (12) | N2–C21–C22 | 122.90 (16) |
| O2–C23–C24 | 118.23 (14) | O2–C23–C22 | 121.32 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Carbón, P.; Fernández-Fariña, S.; Martínez-Calvo, M. Design, Synthesis, and Structural Study of a Shiff Base Ligand Precursor of Metallosupramolecular Architectures. Chem. Proc. 2024, 16, 70. https://doi.org/10.3390/ecsoc-28-20142
Domínguez-Carbón P, Fernández-Fariña S, Martínez-Calvo M. Design, Synthesis, and Structural Study of a Shiff Base Ligand Precursor of Metallosupramolecular Architectures. Chemistry Proceedings. 2024; 16(1):70. https://doi.org/10.3390/ecsoc-28-20142
Chicago/Turabian StyleDomínguez-Carbón, Paula, Sandra Fernández-Fariña, and Miguel Martínez-Calvo. 2024. "Design, Synthesis, and Structural Study of a Shiff Base Ligand Precursor of Metallosupramolecular Architectures" Chemistry Proceedings 16, no. 1: 70. https://doi.org/10.3390/ecsoc-28-20142
APA StyleDomínguez-Carbón, P., Fernández-Fariña, S., & Martínez-Calvo, M. (2024). Design, Synthesis, and Structural Study of a Shiff Base Ligand Precursor of Metallosupramolecular Architectures. Chemistry Proceedings, 16(1), 70. https://doi.org/10.3390/ecsoc-28-20142







