Benzo[a]phenoxazines as Potential Anti-Inflammatory Drugs †
Abstract
1. Introduction
2. Results and Discussion
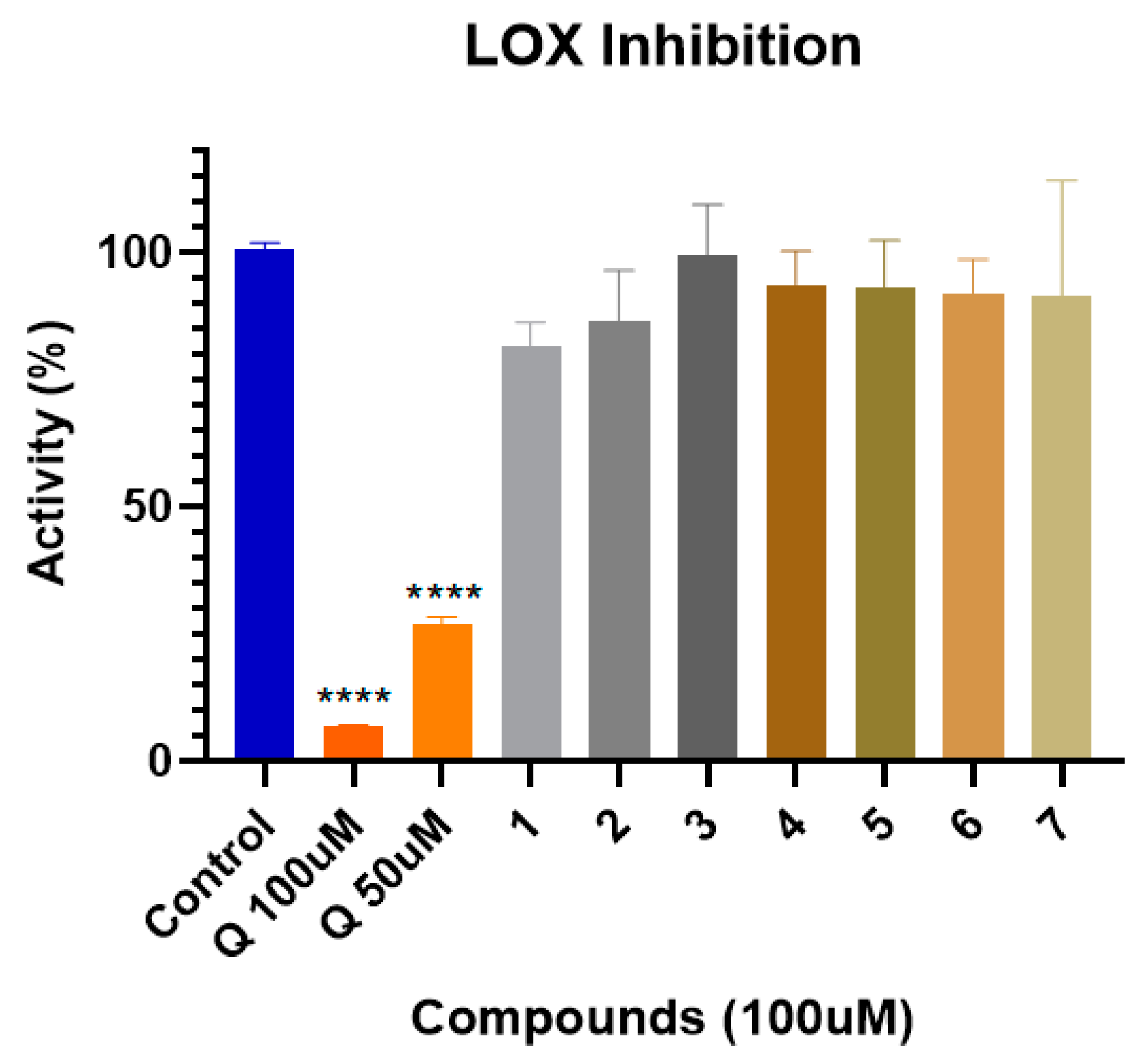
2.1. LOX Inhibition
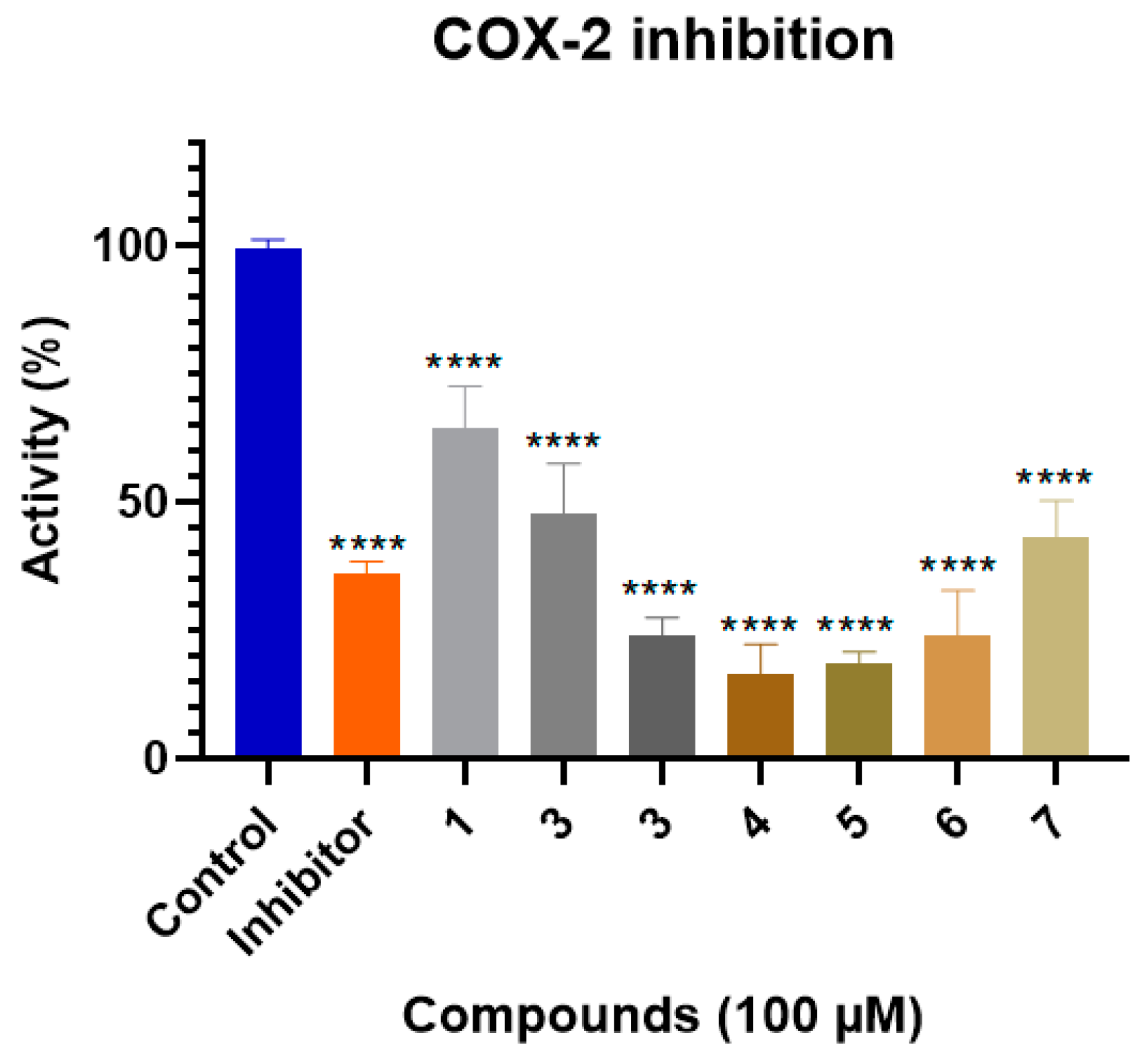
2.2. COX-2 Inhibition
3. Experimental Procedure
3.1. Typical Procedure for LOX Inhibition
3.2. Typical Procedure for COX Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Stürtz, A.R.; Maier, M.; Graham, D.L.; Hirz, M.; Reichelt, M.; Mack, M.; König, W.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef]
- Hajdú, I.; Kardos, J.; Major, B.; Fabó, G.; Lőrincz, Z.; Cseh, S.; Dormán, G. Inhibition of the LOX enzyme family members with old and new ligands. Selectivity analysis revisited. Bioorganic Med. Chem. Lett. 2018, 28, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Langenbach, R. Why there are two cyclooxygenase isozymes. J. Clin. Invest. 2001, 107, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem. Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Jose, J.; Ueno, Y.; Burgess, K. Water-soluble Nile Blue derivatives: Syntheses and photophysical properties. Chemistry 2009, 5, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Odin, E.M.; Onojah, P.K.; Akpanisi, L.E.S.; Akabueze, B.O. Synthesis of Pent-aza Phenoxazines: A New Group of Anti-inflammatory Heterocycles. Their Effect and Other Analgesics on Pain. ACSJ 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Katsamakas, S.; Zografos, A.L.; Vasiliki, S. Advances of Phenoxazines: Synthesis, Reactivity and Their Medicinal Applications. Curr. Med. Chem. 2016, 23, 2972–2999. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Dwivedi, J.; Yaduvanshi, N.; Jain, S. Medicinal and Biological Significance of Phenoxazine Derivatives. Mini Rev. Med. Chem. 2021, 21, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, G.A.; Patrono, C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001, 345, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Y.; Sabir, J.S.M.; Qureshi, M.I.; Saini, K.S. 5-Lipoxygenase: A Promising Drug Target Against Inflammatory Diseases-Biochemical and Pharmacological Regulation. Curr. Drug Targets 2014, 15, 410–422. [Google Scholar] [CrossRef]
- Warner, T.D.; Mitchell, J.A. Cyclooxygenases: New forms, new inhibitors, and lessons from the clinic. FASEB J. 2004, 18, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.H.J.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Moura, J.C.V.P. Synthesis and spectral properties of long-wavelength fluorescent dye. J. Photochem. Photobiol. A Chem. 2007, 185, 220–230. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Coutinho, P.J.G.; Moura, J.C.V.P.; Gonçalves, M.S.T. Functionalised benzo[a]phenoxazine dyes as long-wavelength fluorescent probes for amino acids. Tetrahedron 2007, 63, 1654–1663. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Gonçalves, M.S.T.; Pereira, D.M. Benzo[a]phenoxazines as Potential Anti-Inflammatory Drugs. Chem. Proc. 2024, 16, 71. https://doi.org/10.3390/ecsoc-28-20167
Pinto J, Gonçalves MST, Pereira DM. Benzo[a]phenoxazines as Potential Anti-Inflammatory Drugs. Chemistry Proceedings. 2024; 16(1):71. https://doi.org/10.3390/ecsoc-28-20167
Chicago/Turabian StylePinto, Joana, M. Sameiro T. Gonçalves, and David M. Pereira. 2024. "Benzo[a]phenoxazines as Potential Anti-Inflammatory Drugs" Chemistry Proceedings 16, no. 1: 71. https://doi.org/10.3390/ecsoc-28-20167
APA StylePinto, J., Gonçalves, M. S. T., & Pereira, D. M. (2024). Benzo[a]phenoxazines as Potential Anti-Inflammatory Drugs. Chemistry Proceedings, 16(1), 71. https://doi.org/10.3390/ecsoc-28-20167







