Organocatalytic Cascade Reactions for the Synthesis and Diversification of Privileged Structures †
Abstract
1. Introduction
2. Methods
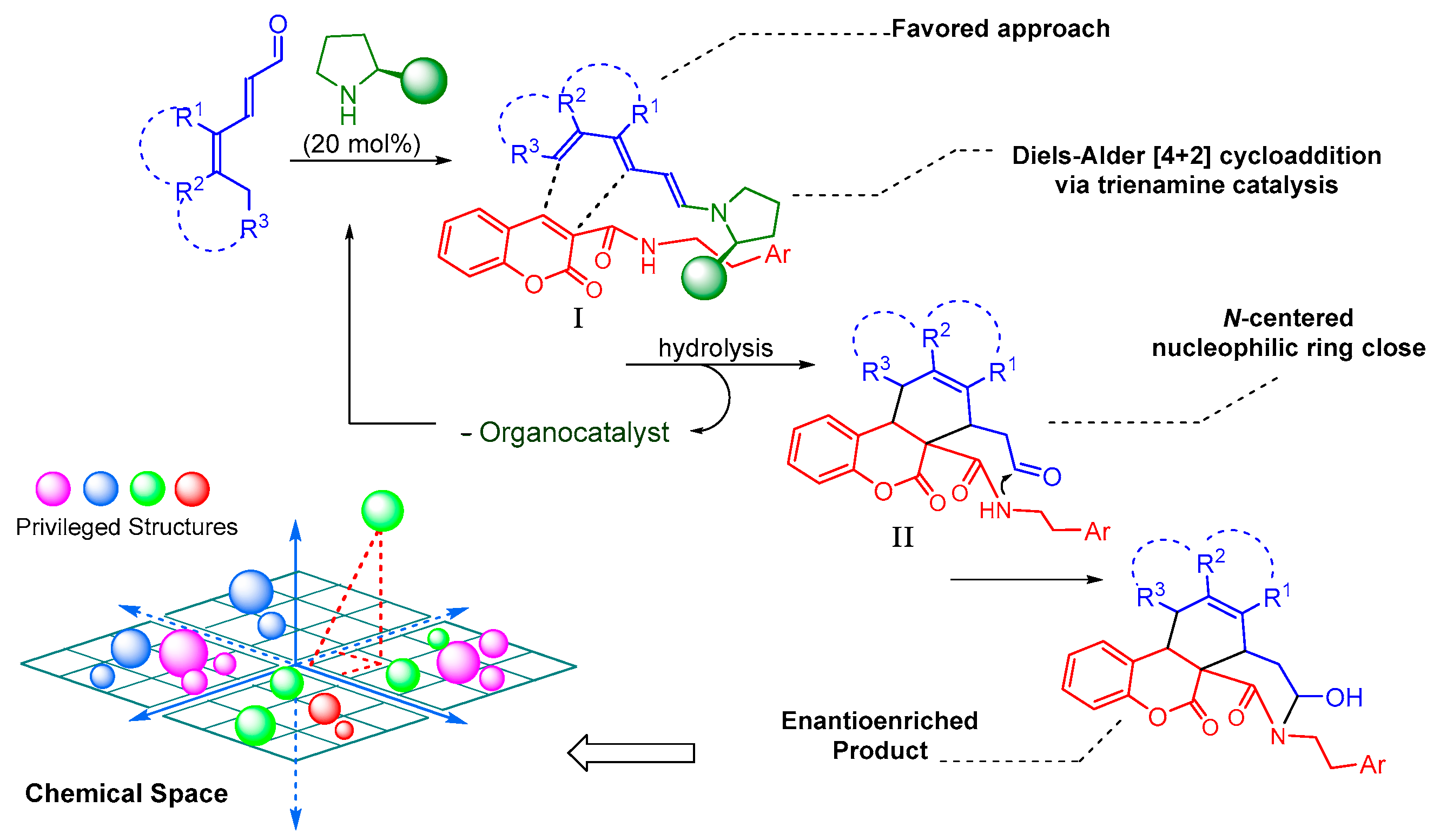
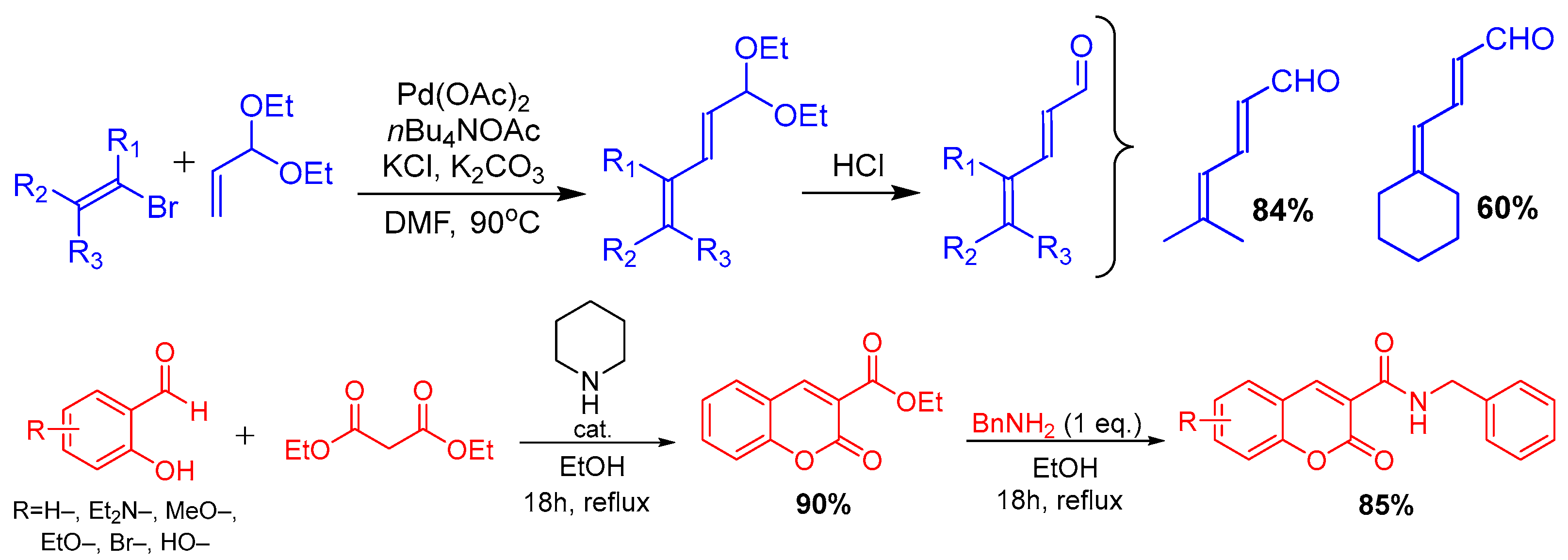
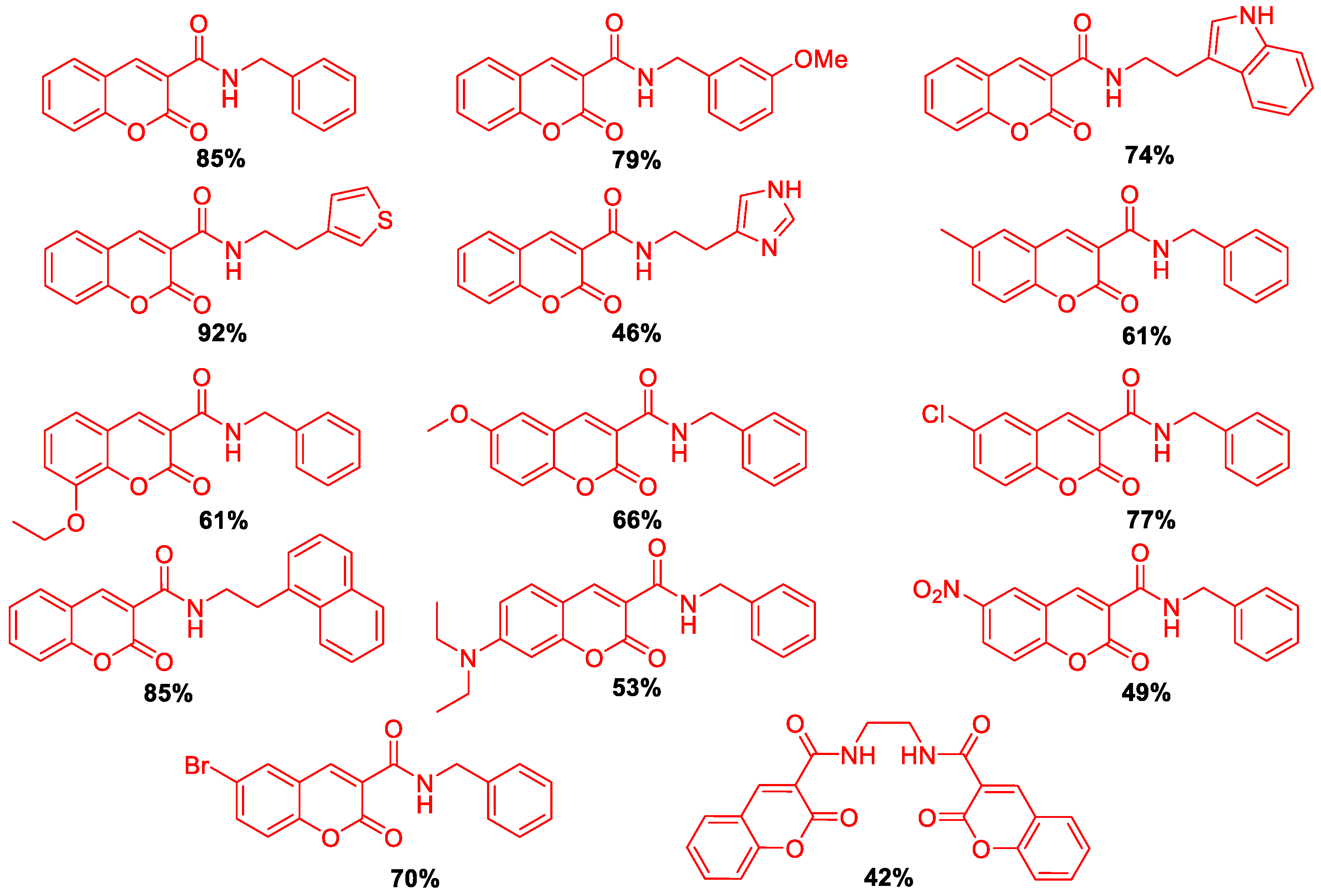
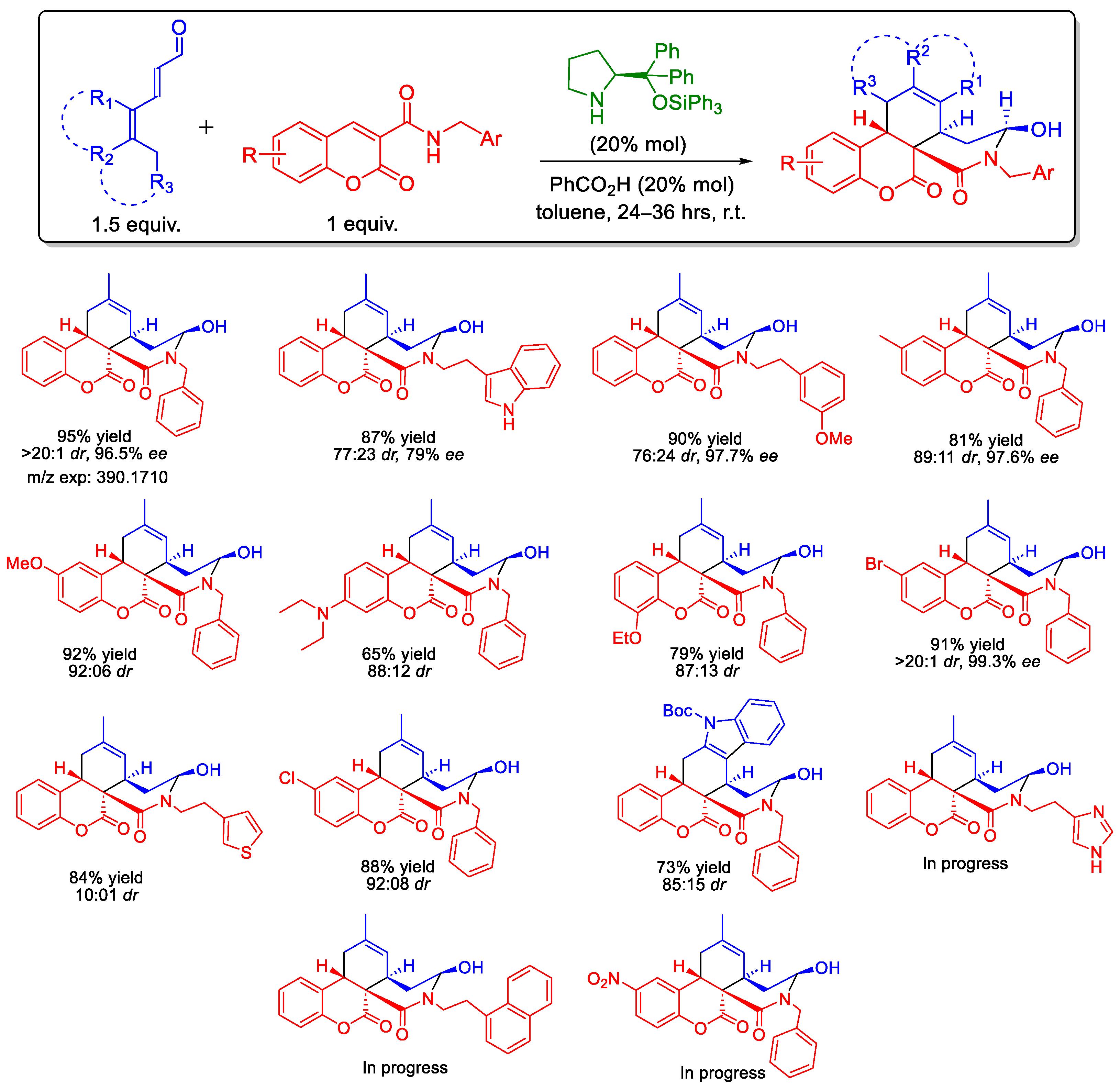
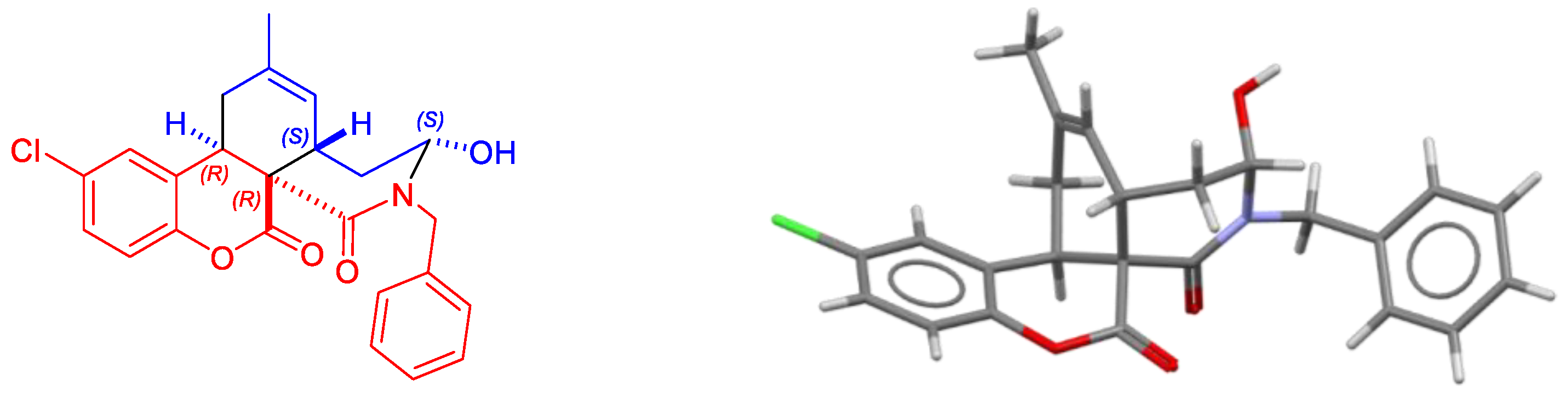
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawar, T.J.; Jiang, H.; Vázquez-Guevara, M.A.; Villegas-Gómez, C.; Cruz-Cruz, D. Aminocatalytic Privileged Diversity-Oriented Synthesis (ApDOS): An Efficient Strategy to Populate Relevant Chemical Spaces. Eur. J. Org. Chem. 2018, 16, 1835–1851. [Google Scholar] [CrossRef]
- Gómez, C.V.; Cruz, D.C.; Mose, R.; Jørgensen, K.A. Organocatalytic cascade reactions: Diversity-oriented synthesis for the construction of hydroisoquinoline scaffolds. Chem. Commun. 2014, 50, 6035. [Google Scholar] [CrossRef] [PubMed]
- Mitkari, S.B.; Medina-Ortíz, A.; Olivares-Romero, J.; Vázquez-Guevara, M.A.; Peña-Cabrera, E.; Villegas-Gómez, C.; Cruz-Cruz, D. Organocatalytic cascade reactions for the diversification of thiopyrano-piperidone fused rings utilizing trienamine activation. Chem. Eur. J. 2020, 27, 618–621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortíz, A.M.; Cruz, D.C.; Gómez, C.V. Organocatalytic Cascade Reactions for the Synthesis and Diversification of Privileged Structures. Chem. Proc. 2024, 16, 72. https://doi.org/10.3390/ecsoc-28-20136
Ortíz AM, Cruz DC, Gómez CV. Organocatalytic Cascade Reactions for the Synthesis and Diversification of Privileged Structures. Chemistry Proceedings. 2024; 16(1):72. https://doi.org/10.3390/ecsoc-28-20136
Chicago/Turabian StyleOrtíz, Alberto Medina, David Cruz Cruz, and Clarisa Villegas Gómez. 2024. "Organocatalytic Cascade Reactions for the Synthesis and Diversification of Privileged Structures" Chemistry Proceedings 16, no. 1: 72. https://doi.org/10.3390/ecsoc-28-20136
APA StyleOrtíz, A. M., Cruz, D. C., & Gómez, C. V. (2024). Organocatalytic Cascade Reactions for the Synthesis and Diversification of Privileged Structures. Chemistry Proceedings, 16(1), 72. https://doi.org/10.3390/ecsoc-28-20136





