Microwave Activation: Highly Efficient Hydrolysis of Hesperidin and Naringin and Synthesis of Their Aglycone Acetates Under Microwave Irradiation †
Abstract
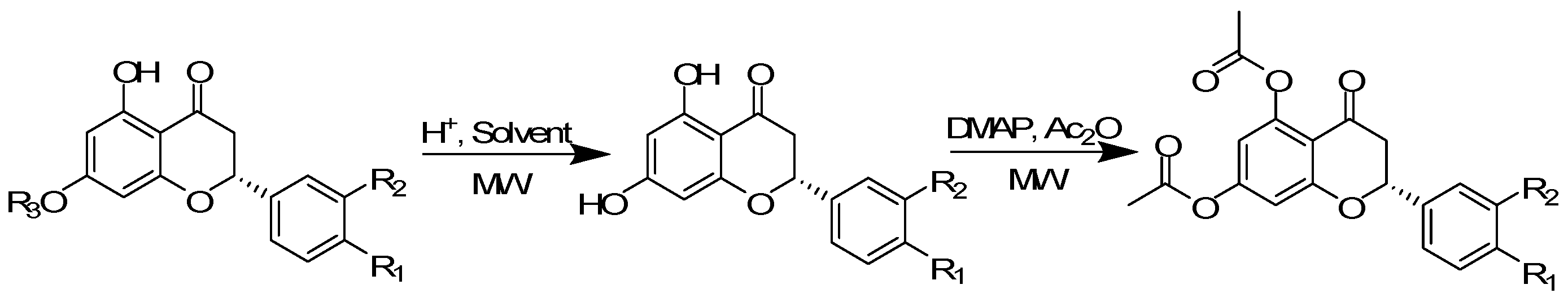
1. Introduction
2. Results and Discussion
3. Experimental
3.1. Extractions of Flavonoids
3.2. O1 and Naringin 2
3.3. Catalytic Esterification of Flavanones 2–5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikans, R. Natural Compounds: An Laboratory Guide; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Putignani, L.; Massa, O.; Alisi, A. Engineered Escherichia coli as new source of flavonoids and terpenoids. Food Res. Int. 2013, 54, 1084–1095. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-Q.; Chen, J.-C.; Liu, D.-H.; Jiang, P.; Shi, J.; Xue, S.; Wu, D.; Xu, J.-G.; Kakuda, Y. Identification of bioactive composition and antioxidant activity in young mandarin fruits. Food Chem. 2011, 124, 1561–1566. [Google Scholar] [CrossRef]
- Nazari, M.; Ghorbani, A.; Hekmat-Doost, A.; Jeddi-Tehrani, M.; Zand, H. Inactivation of Nuclear Factor-κB by citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in Ramos cells. Eur. J. Pharmacol. 2011, 650, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K. Imbalance in antioxidant defense and human diseases: Multiple approach of natural antioxidants therapy. Curr. Sci. 2001, 81, 1179–1187. [Google Scholar]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Hesperidin plays beneficial roles in disorders associated with the central nervous system: A review. Int. J. Food Prop. 2023, 26, 1867–1884. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Höfle, G.; Steglich, W.; Vorbrüggen, H. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. [New synthetic method (25)]. Angew. Chem. Int. Ed. Engl. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fandougouma, O.; Cheikh, N.; Villemin, D.; Bar, N. Microwave Activation: Highly Efficient Hydrolysis of Hesperidin and Naringin and Synthesis of Their Aglycone Acetates Under Microwave Irradiation. Chem. Proc. 2024, 16, 65. https://doi.org/10.3390/ecsoc-28-20187
Fandougouma O, Cheikh N, Villemin D, Bar N. Microwave Activation: Highly Efficient Hydrolysis of Hesperidin and Naringin and Synthesis of Their Aglycone Acetates Under Microwave Irradiation. Chemistry Proceedings. 2024; 16(1):65. https://doi.org/10.3390/ecsoc-28-20187
Chicago/Turabian StyleFandougouma, Omar, Nawel Cheikh, Didier Villemin, and Nathalie Bar. 2024. "Microwave Activation: Highly Efficient Hydrolysis of Hesperidin and Naringin and Synthesis of Their Aglycone Acetates Under Microwave Irradiation" Chemistry Proceedings 16, no. 1: 65. https://doi.org/10.3390/ecsoc-28-20187
APA StyleFandougouma, O., Cheikh, N., Villemin, D., & Bar, N. (2024). Microwave Activation: Highly Efficient Hydrolysis of Hesperidin and Naringin and Synthesis of Their Aglycone Acetates Under Microwave Irradiation. Chemistry Proceedings, 16(1), 65. https://doi.org/10.3390/ecsoc-28-20187





