1. Introduction
An enteric coating is a barrier polymer applied on pharmaceutical oral dosage forms with gastric resistance properties [
1]. The coatings prepared from such polymers remain intact in gastric fluid but disintegrate in the small intestine [
2]. An enteric phase indicates the small intestine; therefore, these coatings prevent the release content of solid pharmaceutical dosage forms (tablets, pellets, and granules) before they reach the small intestine. Several reasons exist for using such a coating on oral pharmaceutical formulations [
3], such as to protect the stomach from drugs that irritate the stomach, for example, aspirin and diclofenac. The focus is on protecting the drugs from the stomach, which are unstable in an acidic environment. This prevents drug release in the stomach but dissolves in the intestine. The polymers that are used for gastro-resistance applications are pH-sensitive polymers, which remain unionised at low pH~3.
For this reason, they do not dissolve in the stomach. In contrast, in the more neutral pH, 6.8–7 in the small intestine, the carboxylic group can become ionised, allowing the coating to dissolve; after that, the drug will be released [
4]. The gastrointestinal tract (GIT) has physiological factors that might affect the function of enteric coating. These factors, which were reported by [
1,
5], include the pH of the stomach and intestinal content, gastric emptying, and the enzyme activity of the GIT. The gastric pH variable from 1 to 3.5 depends on the feeding status and reflux of intestinal content into the stomach, whereas the pH of the small intestine varies from about 3.8 to 6.8 and extends to 7.5–8 in the large intestine. According to these pH values, the enteric coating must be designed to avoid dissolution at pH values below 4 to resist disintegration in the stomach. Currently, the enteric substances that have been used are typically synthetic or semi-synthetic polymers. The term polymer can be defined as a large molecule with high molecular weight composed of many simpler molecules. These molecules are linked together to form a long chain. Furthermore, it can occur naturally or synthetically [
6]. For many years, polymers have been used for pharmaceutical applications, and recently, they have played a significant role in the fabrication of many delayed-release and drug-targeting site formulations. Before embarking on polymeric coating, one can identify the types of polymers and how they are made or prepared. Polymers are classified into three main categories. The first is natural polymers, which occur in nature and require purification processes; however, they do not need any chemical modification, such as shellac, alginate, pectin, and chitosan. The second category is known as semi-synthetic polymers. This kind of polymer is derived from natural materials and requires chemical modification. Cellulose derivatives are one example of such polymers.
In contrast, the third category is synthetic polymers, fully chemically synthesised, such as methacrylic acid copolymers [
7]; polymers have a long history in pharmaceutical practice. The first enteric coating of natural origin, shellac, was introduced by Nun in 1884. However, the application of shellac as an enteric coating has been limited due to its poor water solubility [
8]. Therefore, this type of polymer was not reliable to use as an enteric coating.
Whey-isolated protein (WIP) is another biodegradable protein used for therapeutic purposes. In the current project, two natural polymers (soy protein isolate and whey protein isolate) can be used as an enteric coating for pharmaceutical dosage forms. Although these natural coating systems have already been applied to delayed release, there is no well-known literature about their performance under gastric conditions or to compare it with other studies [
9]. Whey proteins, along with casein, are one of the main clusters of proteins in milk.
2. Experimental Procedure
Film preparation: films were cast from soy protein isolate (SPI) and whey protein isolate (WPI) using organic solvents such as HCl, ethanol, acetic acid, and isopropanol. These films were evaluated for their resistance to acidic pH through disintegration tests and examined using scanning electron microscopy (SEM).
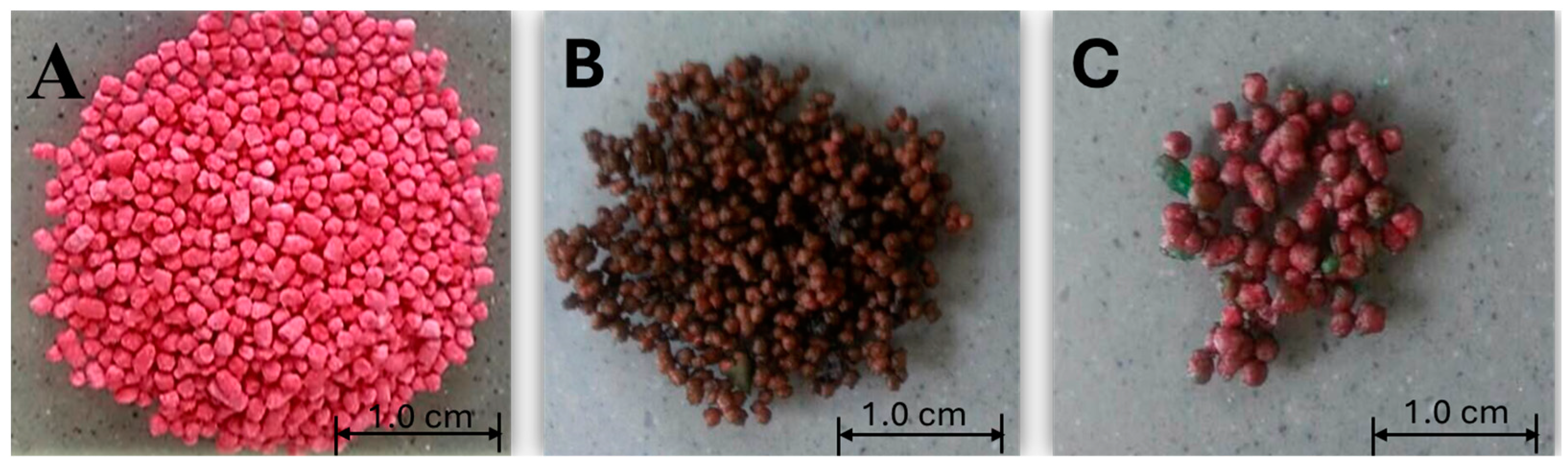
Pellet preparation followed the method proposed by (Sinha, Agrawal, & Kumria, 2005). The powders contained three ingredients (15 g of Avicel® PH 101, 5 g of lactose and 40 mg of red pigment). These ingredients were mixed in dry form using a Caleva mixer. The pellets were produced using the spherometer–extruder. These pellets were coloured by adding a red pigment. There are two reasons for adding red pigment instead of adding medicine. The first was to detect the total amount of red colour through the wavelength absorbed by the solution. Therefore, only the photons travelling at 514 nm are reflected or absorbed back into the spectrum. Second, when determining the pigment amount that is released in the solution, it will be easy to evaluate the performance of the coating films.
Coating procedure: this was performed using spray-coating equipment (Caleva Multi lab, UK). Three grams of pellets were coated with SAG solution, and the same amount of pallets were coated with SA solution. The dried pellets were loaded in a vibrating coating chamber. The coating solutions were sprayed on the pellets. After coating, the pellets were oven-dried at 60 °C for 1 h. The coating process was performed separately; the resulting pellets are shown in
Figure 1.
Percentages of red pigment inside the uncoated and coated pellets were used to calculate the percentage of pigment released from the pellets because the experimental amounts were more accurate than theoretical amounts. These amounts and percentages are shown in
Table 1.
Differential Scanning Calorimetry (DSC): the thermal properties of the films were analysed using DSC. The films were heated from 25 °C to 250 °C at 10 °C/min under a nitrogen atmosphere to prevent mass loss.
Disintegration test: films were tested for gastro-resistance by placing them in 0.1 M HCl (pH 1.2) for 2 h, followed by exposure to a phosphate buffer (pH 6.8) for 45 min. This test was conducted in a shaking water bath to simulate gastrointestinal conditions.
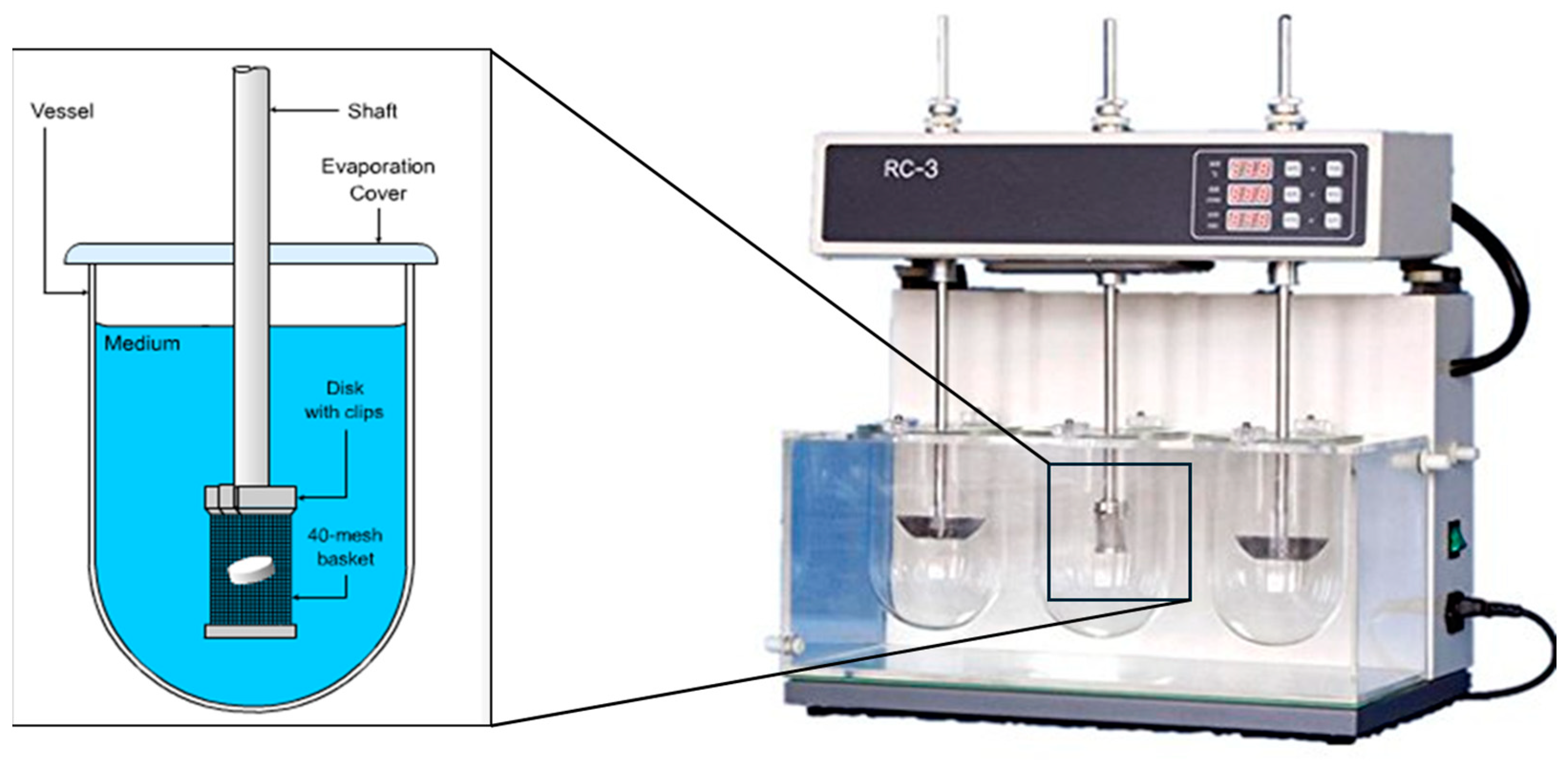
Dissolution testing: in vitro dissolution studies were conducted using a USP dissolution apparatus, as shown in
Figure 2. The coated pellets were tested in 0.1 M hydrochloric acid (pH 1.2) for 2 h, followed by a phosphate-buffered solution (pH 6.8) for 45 min. The release rates were measured to evaluate the gastro-resistance properties of the coatings.
3. Results and Discussion
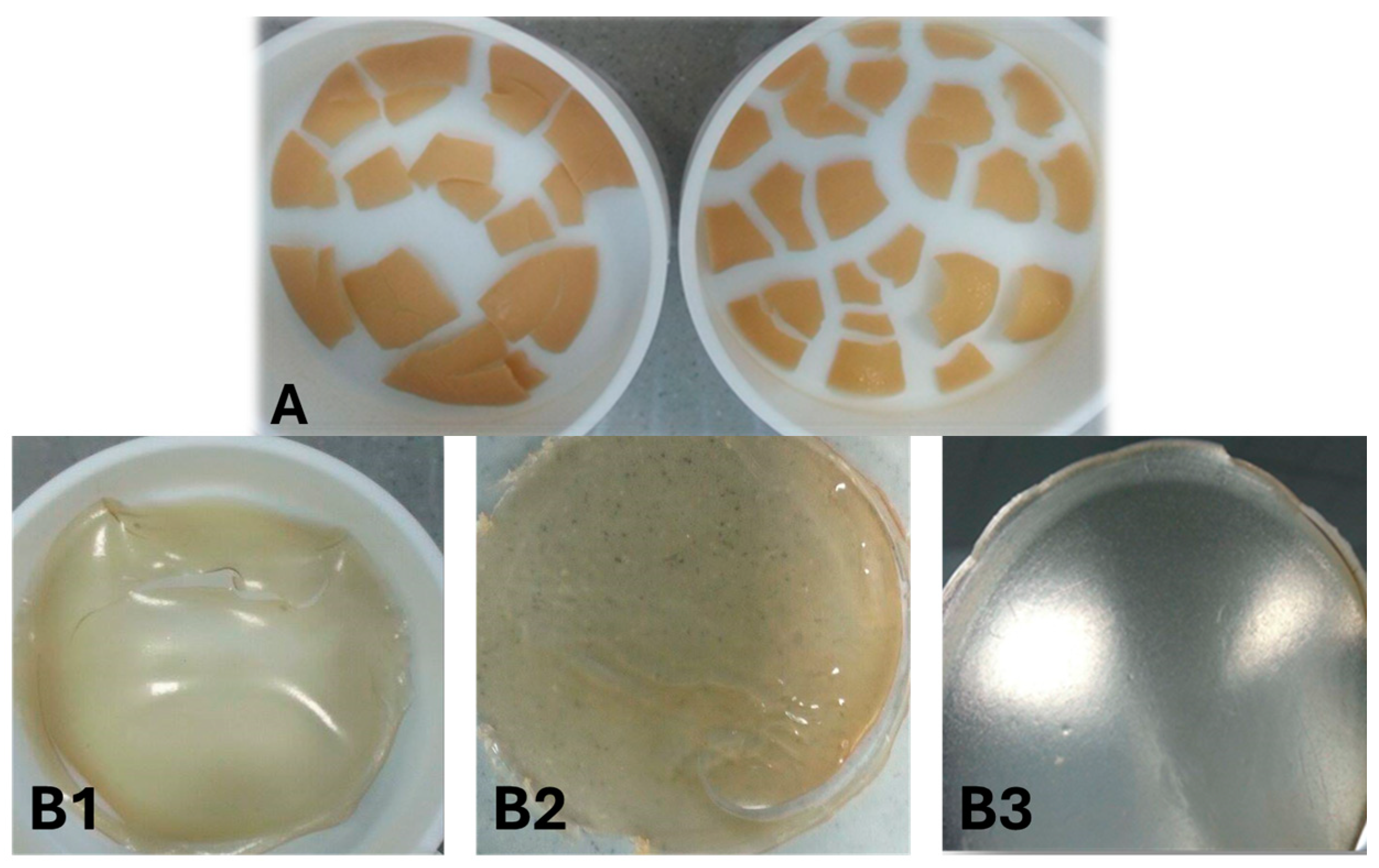
The physical characteristics of SPI and WPI films: films (ST1 and ST2) prepared in present aqueous ethanol have a non-free-standing film despite plasticisers (Tec). This is possible because the ethanol is not compatible with SPI. However, water at 100% and HCl (0.1 M or 0.2 M) as a solvent produced a complete and non-cracked film (for example, SA1, SA2, and SA3), as shown in
Figure 2. WPI films produced gel when adding 0.2 M HCl to the WPI/ethanol solution. This might be due to changes in pH and the heating effect. All films dissolved in the acid phase, meaning these films do not have acid resistance properties and are very sensitive to acids.
This study evaluated the physical characteristics of whey protein isolate (WPI) and soy protein isolate (SPI) films, focusing on their solvent compatibility, plasticiser use, and thermal treatment. The thermal treatment of SPI is a well-established method used to enhance its solubility by denaturing the protein, which involves breaking disulfide bonds. This process improves the functional properties of SPI, making it more suitable for coating applications.
Protein denaturation and solubility: the denaturation of proteins, such as SPI, occurs when their native structure is altered without changing the amino acid sequence, typically due to exposure to different pH levels or temperatures. Heating soy protein can segregate subunits into more minor molecular weight compounds, which may enhance protein solubility.
For hydrolysis and molecular breakdown, the degree of protein hydrolysis increases with temperature, accelerating the breakdown of protein molecules in SPI films. This reaction is crucial for improving the film’s characteristics, potentially making it more effective as a coating material.
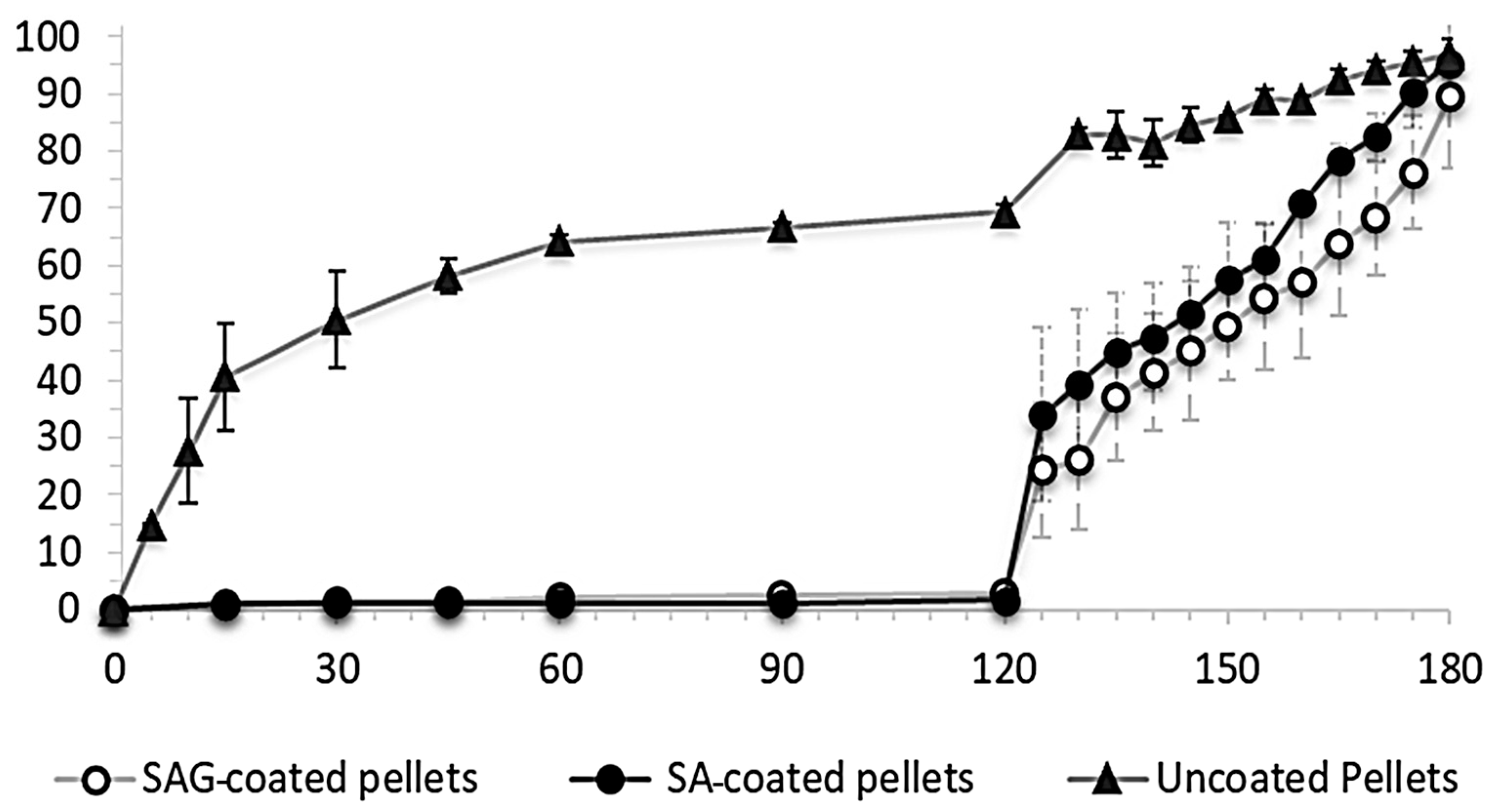
Enteric coating development: a slight dissolution occurred for 120 min (acid phase) for both coated pellets (SA and SAG). The dye percentage release was only 1% for coated pellets (SA) and approximately 2% for coated pellets (SAG). However, when adding phosphate buffer of pH 6.8 after 5–10 min, the percentage release was increased significantly by over 34% and roughly 24% for SA- and SAG-coated pellets, respectively. Furthermore, the percentage releases reached 95% (SA) and 90% (SAG) within 180 min following the buffer phase.
The coated pellets illustrated excellent physical resistance to the acid phase, with the acid uptake value between 1 and 2% in the first two hours, followed by modifying to basic by adding phosphate buffer pH 6.8 to cause rapid dissolution of a coating film. As shown in
Figure 3, the SAG-coated pellets dissolved faster than the SA-coated pellets. This is probably because the coating film contains glycerol, which leads to this effect. However, the dissolving rate for pellets coated by SA is much greater than that of coated pellets by SAG during the whole buffer phase with pH 6.8.
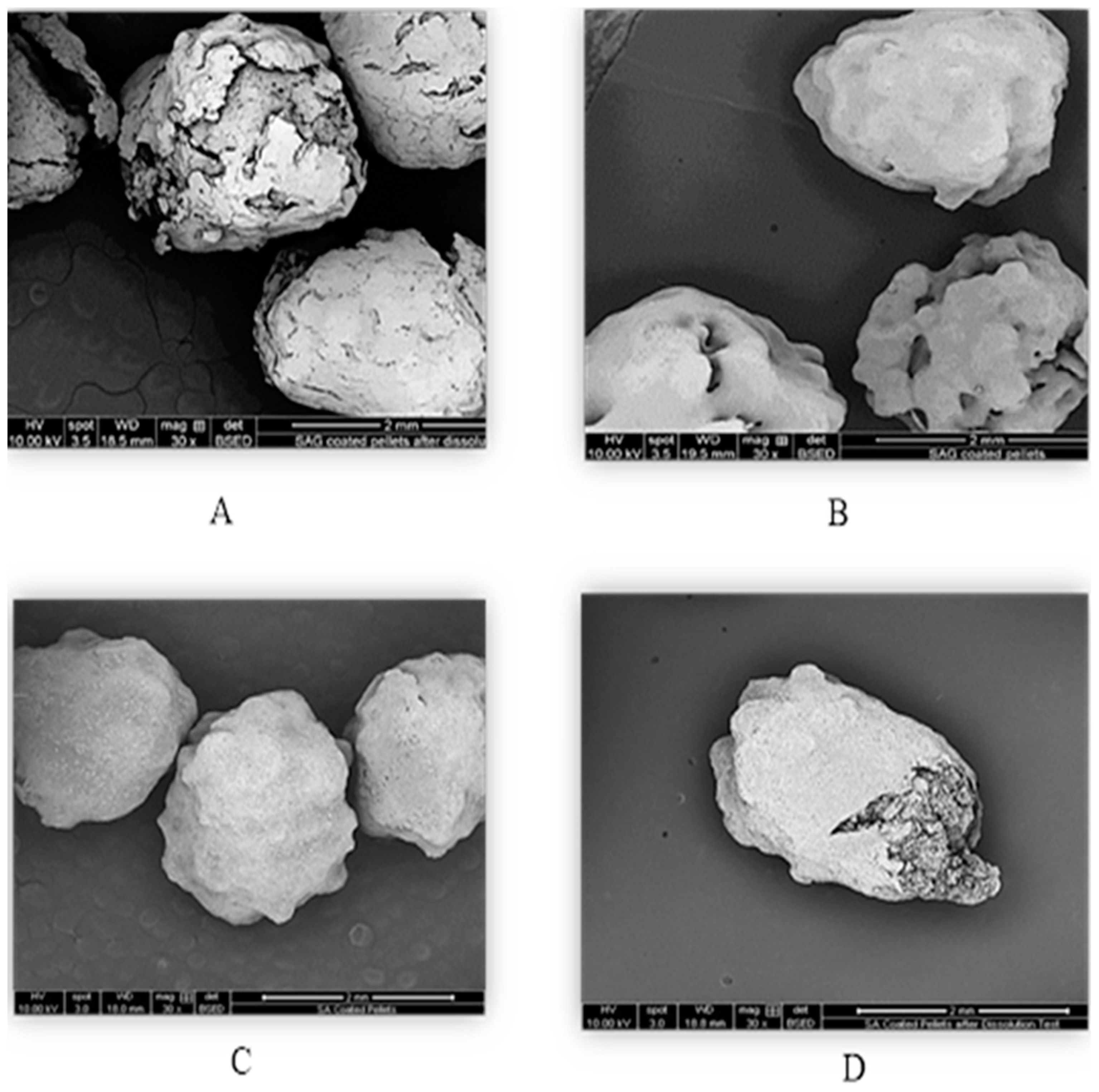
The development of enteric coatings using natural polymers like soy protein shows promise. The two-coating films from SPI demonstrated gastrointestinal resistance, remaining intact for 2 h in acidic pH and releasing the dye under intestinal conditions at pH 6.8. This indicates the potential of SPI films for delayed-release applications. SEM images illustrate the surface morphology of pellets before and after the dissolution test. It is clear in
Figure 4 that pellets coated with SAG ruptured more than SA pellets. The disintegration of the coating film for the pellets coated with SA was not complete. According to USP criteria, for a successful entering coating, the percentage release should be less than 10% in the first 2 h in the acidic phase, followed by at least 80% of the percentage release within 45 min in the buffer phase. The coating films met the USP-specific limit for enteric coating films at 45 min. The percentage of dye release was 78.534% ± DS. After 5 min, the rate increased to 82%. This percentage is considered a significant value. The SEM images as shown in
Figure 5 demonstrate the shape of both SAG and SA coated pellets before and after the changes associated with dissolution test.
4. Conclusions
As an initial start, this study primally developed a two-layer coating film using natural soy and whey proteins, demonstrating promising potential for delayed-release applications. The dissolution experiments revealed that the SA and SAG films maintained their integrity in acidic conditions for up to 2 h and effectively released the dye under intestinal conditions at pH 6.8. These findings suggest that such protein-based coatings could benefit pharmaceutical dosage forms, offering advantages as dietary supplements and nutraceutical products. However, the formulations using whey protein alone or blended with zein protein did not yield successful results, indicating a need for further research to optimise enteric coatings from protein-based films like WPI and SPI. Overall, this study focuses on the potential of natural polymers as viable alternatives to synthetic polymers for enteric coatings, although challenges that require additional investigation remain. Despite the promising results, this study acknowledges limitations in the current study. The formulations using whey protein alone or blended with zein protein were studied. Future research is needed to optimise protein-based films like WPI and SPI for enteric coating applications.
Author Contributions
Methodology, E.B.; validation and testing E.B.; data analysis, E.B., A.S., S.T. and M.M.; investigation, E.B. and M.M.; resources, E.B. and A.S.; writing—original draft preparation, E.B.; writing—review E.B., M.M., S.T. and A.S. and editing, E.B., M.M., S.T. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to recognise the assistance provided by North Africa International Center for Research on Serums, Vaccines and Genetic Diseases, Zawia, Libya; Medical Research Center Zawia, Libyan Authority for Scientific Research; as well as the Libyan Scholarship Program and the support from Huddersfield University during this research program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Katona, M.T.; Kakuk, M.; Szabó, R.; Tonka-Nagy, P.; Takács-Novák, K.; Borbás, E. Towards a Better Understanding of the Post-Gastric Behavior of Enteric-Coated Formulations. Pharm. Res. 2022, 39, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Salawi, A. Pharmaceutical Coating and Its Different Approaches, a Review. Polymers 2022, 14, 3318. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Heller, J. Controlled Drug Release from Monolithic Systems. In Ophthalmic Drug Delivery; Springer New York: New York, NY, USA, 1987; Volume 1, pp. 179–189. [Google Scholar]
- Muley, S.; Nandgude, T.; Poddar, S. Extrusion–spheronization a promising pelletization technique: In-depth review. Asian J. Pharm. Sci. 2016, 11, 684–699. [Google Scholar] [CrossRef]
- Siepmann, J.; Faham, A.; Clas, S.-D.; Boyd, B.J.; Jannin, V.; Bernkop-Schnürch, A.; Zhao, H.; Lecommandoux, S.; Evans, J.C.; Allen, C.; et al. Lipids and polymers in pharmaceutical technology: Lifelong companions. Int. J. Pharm. 2019, 558, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.; Leopold, C.S. Development of shellac-coated sustained release pellet formulations. Eur. J. Pharm. Sci. 2011, 42, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-Related Aspects of Milk Proteins. Iranian Journal of Pharmaceutical Research: IJPR. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27980594 (accessed on 25 August 2024).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).