Synthesis and Evaluation of Novel 5-Arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) Thiazolidin-4-Ones as Anti-Microbial Agents †
Abstract
:1. Introduction
2. Materials and Methods
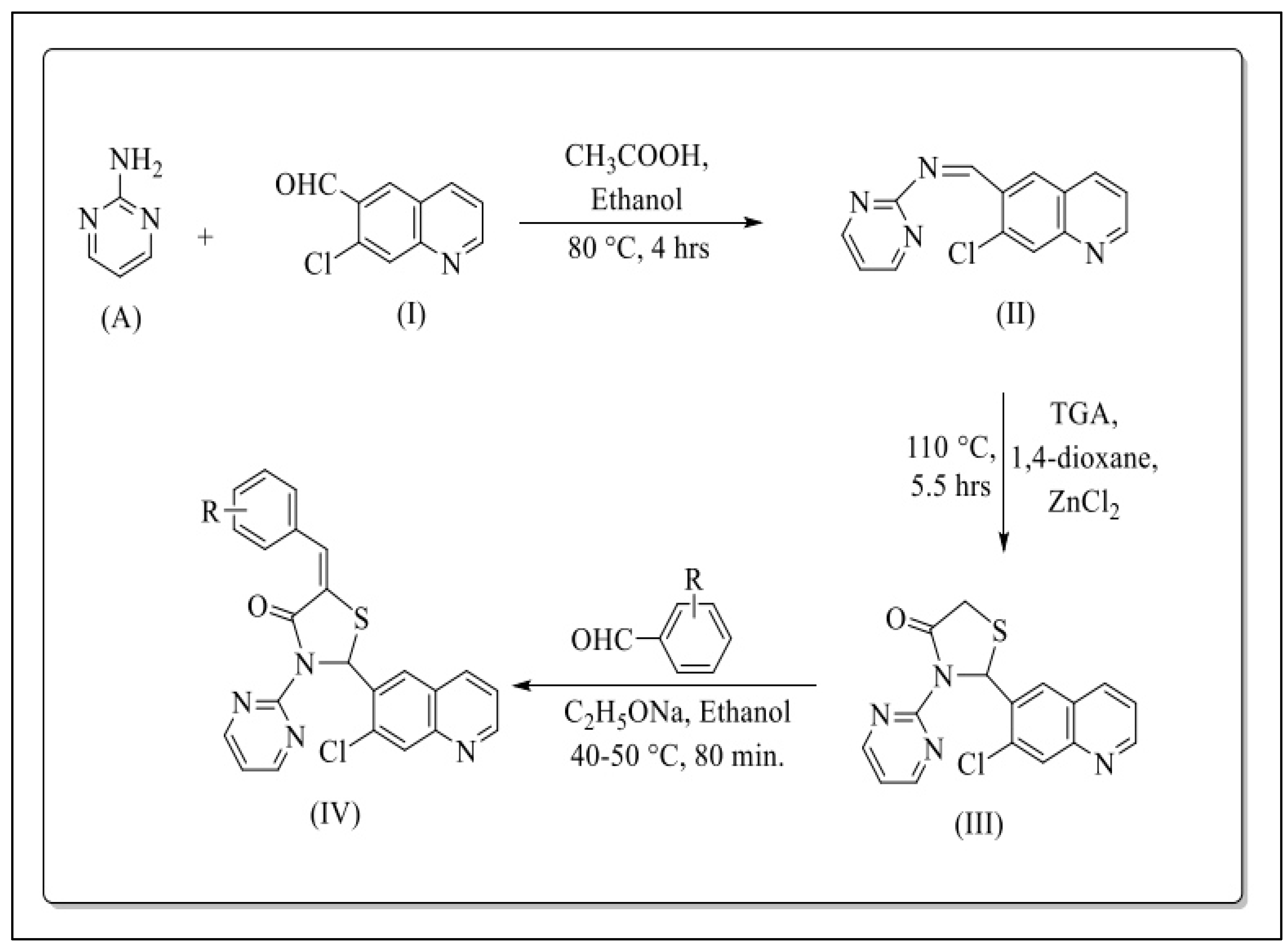
- Preparation of 7-chloroquinoline-6-carbaldehyde (I)
- Preparation of 1-(7-chloroquinolin-6-yl)-N-(pyrimidin-2-yl)methanimine (II)
- Preparation of 2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl)thiazolidin-4-one (III)
- Preparation of 5-arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) thiazolidin-4-ones (IV)
2.1. Biological Evaluation
2.1.1. Antimicrobial Assay
2.1.2. Antibacterial Assay
2.1.3. Antifungal Assay
3. Result and Discussion
3.1. Chemistry
3.2. Biological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of Antibiotics. From Salvarsan to Cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The Future of Antibiotics and Resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- McGowan, J.E., Jr. Economic Impact of Antimicrobial Resistance. Emerg. Infect. Dis. 2001, 7, 286–292. [Google Scholar] [CrossRef]
- Belen’kii, L.I.; Evdokimenkova, Y.B. The Literature of Heterocyclic Chemistry, Part XVII, 2017. Adv. Heterocycl. Chem. 2019, 129, 337–418. [Google Scholar]
- Marson, C.M. Saturated Heterocycles with Applications in Medicinal Chemistry. Adv. Heterocycl. Chem. 2017, 121, 13–33. [Google Scholar]
- Debbabi, M.; Nimbarte, V.D.; Chekir, S.; Chortani, S.; Romdhane, A.; Ben jannet, H. Design and Synthesis of Novel Potent Anticoagulant and Anti-TyrosinasePyranopyrimidines and Pyranotriazolopyrimidines: Insights from Molecular Docking and SAR Analysis. Bioorg. Chem. 2019, 82, 129–138. [Google Scholar] [CrossRef]
- Alam, O.; Khan, S.A.; Siddiqui, N.; Ahsan, W.; Verma, S.P.; Gilani, S.J. Antihypertensive Activity of Newer 1,4-Dihydro-5-Pyrimidine Carboxamides: Synthesis and Pharmacological Evaluation. Eur. J. Med. Chem. 2010, 45, 5113–5119. [Google Scholar] [CrossRef]
- He, H.; Wang, W.; Zhou, Y.; Xia, Q.; Ren, Y.; Feng, J.; Peng, H.; He, H.; Feng, L. Rational Design, Synthesis and Biological Evaluation of 1,3,4-Oxadiazole Pyrimidine Derivatives as Novel Pyruvate Dehydrogenase Complex E1 Inhibitors. Bioorg. Med. Chem. 2016, 24, 1879–1888. [Google Scholar] [CrossRef]
- Dube, P.N.; Bule, S.S.; Ushir, Y.V.; Kumbhare, M.R.; Dighe, P.R. Synthesis of novel 5-methyl pyrazol-3-one derivatives and their in vitro cytotoxic evaluation. Med. Chem. Res. 2015, 24, 1070–1076. [Google Scholar] [CrossRef]
- Elsayed, N.M.Y.; Abou El Ella, D.A.; Serya, R.A.T.; Tolba, M.F.; Shalaby, R.; Abouzid, K.A.M. Design, Synthesis and Biological Evaluation of Indazole–Pyrimidine Based Derivatives as Anticancer Agents with Anti-Angiogenic and Antiproliferative Activities. Medchemcomm 2016, 7, 881–899. [Google Scholar] [CrossRef]
- Dongre, R.S.; Meshram, J.S.; Selokar, R.S.; Almalki, F.A.; Hadda, T. Ben. Antibacterial Activity of Synthetic Pyrido[2,3-d]Pyrimidines Armed with Nitrile Groups: POM Analysis and Identification of Pharmacophore Sites of Nitriles as Important pro-Drugs. New J. Chem. 2018, 42, 15610–15617. [Google Scholar] [CrossRef]
- Dube, P.N.; Mokale, S.N.; Shaikh, S.I.; Patil, Y.; Yadav, B.; Deshmukh, P.; Sabde, S. Synthesis and molecular docking analysis of imidazol-5-one derivatives as anti-HIV NNRTIs. Pharm. Chem. J. 2015, 49, 125–131. [Google Scholar] [CrossRef]
- Gaonkar, S.; Savanur, M.A.; Sunagar, M.G.; Puthusseri, B.; Deshapande, N.; Nadaf, A.A.; Khazi, I.A.M. Exploring the Potential of Newly Synthesized 4-Methyl-6-Morpholino-Pyrimidine Derivatives as Antiproliferative Agents. New J. Chem. 2018, 42, 2790–2803. [Google Scholar] [CrossRef]
- Banothu, J.; Khanapur, M.; Basavoju, S.; Bavantula, R.; Narra, M.; Abbagani, S. Synthesis, Characterization and Biological Evaluation of Fused Thiazolo[3,2-a]Pyrimidine Derivatives. RSC Adv. 2014, 4, 22866–22874. [Google Scholar] [CrossRef]
- Grundon, M.F. Quinoline, Quinazoline, and Acridone Alkaloids. Nat. Prod. Rep. 1990, 7, 131–138. [Google Scholar] [CrossRef]
- Dube, P.N.; Bule, S.S.; Mokale, S.N.; Kumbhare, M.R.; Dighe, P.R.; Ushir, Y.V. Synthesis and biological evaluation of substituted 5-methyl-2-phenyl-1H-pyrazol-3(2H)-one derivatives as selective COX-2 inhibitors: Molecular docking study. Chem. Boil. Drug Design 2014, 84, 409–419. [Google Scholar] [CrossRef]
- Dube, P.N.; Waghmare, M.N.; Mokale, S.N. Synthesis, In Vitro, and In Vivo Biological Evaluation and Molecular Docking Analysis of Novel 3-(3-oxo-substitutedphenyl-3-)4-(2-(piperidinyl)ethoxy)phenyl) propyl)-2H-chromen-2-one Derivatives as Anti-breast Cancer Agents. Chem. Boil. Drug Design 2016, 87, 608–617. [Google Scholar] [CrossRef]
- Kamal, A.; Rahim, A.; Riyaz, S.; Poornachandra, Y.; Balakrishna, M.; Kumar, C.G.; Hussaini, S.M.A.; Sridhar, B.; Machiraju, P.K. Regioselective Synthesis, Antimicrobial Evaluation and Theoretical Studies of 2-Styryl Quinolines. Org. Biomol. Chem. 2015, 13, 1347–1357. [Google Scholar] [CrossRef]
- Desai, N.C.; Patel, B.Y.; Dave, B.P. Synthesis and Antimicrobial Activity of Novel Quinoline Derivatives Bearing Pyrazoline and Pyridine Analogues. Med. Chem. Res. 2017, 26, 109–119. [Google Scholar] [CrossRef]
- Sridhar, P.; Alagumuthu, M.; Arumugam, S.; Reddy, S.R. Synthesis of QuinolineAcetohydrazide-Hydrazone Derivatives Evaluated as DNA Gyrase Inhibitors and Potent Antimicrobial Agents. RSC Adv. 2016, 6, 64460–64468. [Google Scholar] [CrossRef]
- Nayak, N.; Ramprasad, J.; Dalimba, U. Synthesis and Antitubercular and Antibacterial Activity of Some Active Fluorine Containing Quinoline–Pyrazole Hybrid Derivatives. J. Fluor. Chem. 2016, 183, 59–68. [Google Scholar] [CrossRef]
- Zhao, M.; Kamada, T.; Takeuchi, A.; Nishioka, H.; Kuroda, T.; Takeuchi, Y. Structure–Activity Relationship of IndoloquinolineAnalogs Anti-MRSA. Bioorg. Med. Chem. Lett. 2015, 25, 5551–5554. [Google Scholar] [CrossRef] [PubMed]
- Dolan, N.; Gavin, D.P.; Eshwika, A.; Kavanagh, K.; McGinley, J.; Stephens, J.C. Synthesis, Antibacterial and Anti-MRSA Activity, in Vivo Toxicity and a Structure–Activity Relationship Study of a Quinoline Thiourea. Bioorg. Med. Chem. Lett. 2016, 26, 630–635. [Google Scholar] [CrossRef]
- Musiol, R. An Overview of Quinoline as a Privileged Scaffold in Cancer Drug Discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef]
- Carmo, A.M.L.; Silva, F.M.C.; Machado, P.A.; Fontes, A.P.S.; Pavan, F.R.; Leite, C.Q.F.; Leite, S.R.d.A.; Coimbra, E.S.; Da Silva, A.D. Synthesis of 4-Aminoquinoline Analogues and Their Platinum(II) Complexes as New Antileishmanial and Antitubercular Agents. Biomed. Pharmacother. 2011, 65, 204–209. [Google Scholar] [CrossRef]
- Eswaran, S.; Adhikari, A.V.; Chowdhury, I.H.; Pal, N.K.; Thomas, K.D. New Quinoline Derivatives: Synthesis and Investigation of Antibacterial and Antituberculosis Properties. Eur. J. Med. Chem. 2010, 45, 3374–3383. [Google Scholar] [CrossRef]
- Mokale, S.N.; Palkar, A.D.; Dube, P.N.; Sakle, N.S.; Miniyar, P.B. Design, synthesis and in vivo screening of some novel quinazoline analogs as anti-hyperlipidemic and hypoglycemic agents. Bioorg. Med. Chem. Lett. 2016, 26, 272–276. [Google Scholar] [CrossRef]
| Sr. No. | R | Chemical Name | Structure |
| 1. | H | 5-(benzylidene)-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) thiazolidin-4-one | 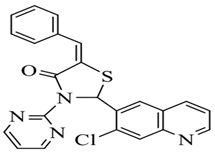 |
| 2. | 4-OCH3 | 5-(4-methoxybenzylidene)-2-(7- chloroquinolin-6-yl)-3-(pyrimidin-2-yl) thiazolidin-4-one | 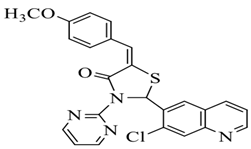 |
| 3. | 4-Cl | 5-(4-chlorobenzylidene)-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) thiazolidin-4-one | 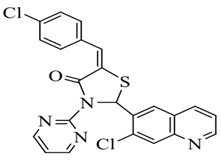 |
| 4. | 4-OH | 5-(4-hydroxybenzylidene)-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) thiazolidin-4-one | 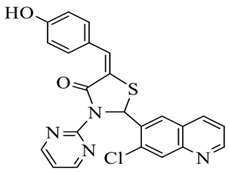 |
| Sr. No. | R | Minimum Inhibitory Concentration (MIC) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria (µg/mL) | Fungi (µg/mL) | |||||||
| E.c. | P.a. | S.a. | S.p. | C.a. | A.n. | A.c. | ||
| 1. | -H | 12.5 | 62.5 | 100 | 200 | 1000 | 250 | 250 |
| 2. | 4-OCH3 | 200 | 12.5 | 500 | 500 | >1000 | 1000 | 1000 |
| 3. | 4-Cl | 250 | 200 | 200 | 200 | 100 | 100 | 62.5 |
| 4. | 4-OH | 12.5 | 200 | 500 | 500 | 250 | 100 | 100 |
| 5. | Gentamicin | 0.05 | 1 | 0.25 | 0.5 | - | - | - |
| 6. | Chloramphenicol | 50 | 50 | 50 | 50 | - | - | - |
| 7. | Ciprofloxacin | 25 | 25 | 50 | 50 | - | - | - |
| 8. | Nystatin | - | - | - | - | 100 | 100 | 100 |
| 9. | Griseofulvin | - | - | - | - | 500 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, P.N.; Thombare, Y.B. Synthesis and Evaluation of Novel 5-Arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) Thiazolidin-4-Ones as Anti-Microbial Agents. Chem. Proc. 2024, 16, 51. https://doi.org/10.3390/ecsoc-28-20120
Dube PN, Thombare YB. Synthesis and Evaluation of Novel 5-Arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) Thiazolidin-4-Ones as Anti-Microbial Agents. Chemistry Proceedings. 2024; 16(1):51. https://doi.org/10.3390/ecsoc-28-20120
Chicago/Turabian StyleDube, Pritam N., and Yogita B. Thombare. 2024. "Synthesis and Evaluation of Novel 5-Arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) Thiazolidin-4-Ones as Anti-Microbial Agents" Chemistry Proceedings 16, no. 1: 51. https://doi.org/10.3390/ecsoc-28-20120
APA StyleDube, P. N., & Thombare, Y. B. (2024). Synthesis and Evaluation of Novel 5-Arylidene-2-(7-chloroquinolin-6-yl)-3-(pyrimidin-2-yl) Thiazolidin-4-Ones as Anti-Microbial Agents. Chemistry Proceedings, 16(1), 51. https://doi.org/10.3390/ecsoc-28-20120






