Kinetic and Equilibrium Analysis of Tartrazine Photocatalytic Degradation Using Iron-Doped Biochar from Theobroma cacao L. Husk via Microwave-Assisted Pyrolysis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining BCCPH-Fe
2.2. Equipments and Characterization
2.3. Experiments and Studies
3. Results and Discussion
3.1. BCCPH-Fe
3.2. BCCPH-Fe Characterization
3.3. Experiments
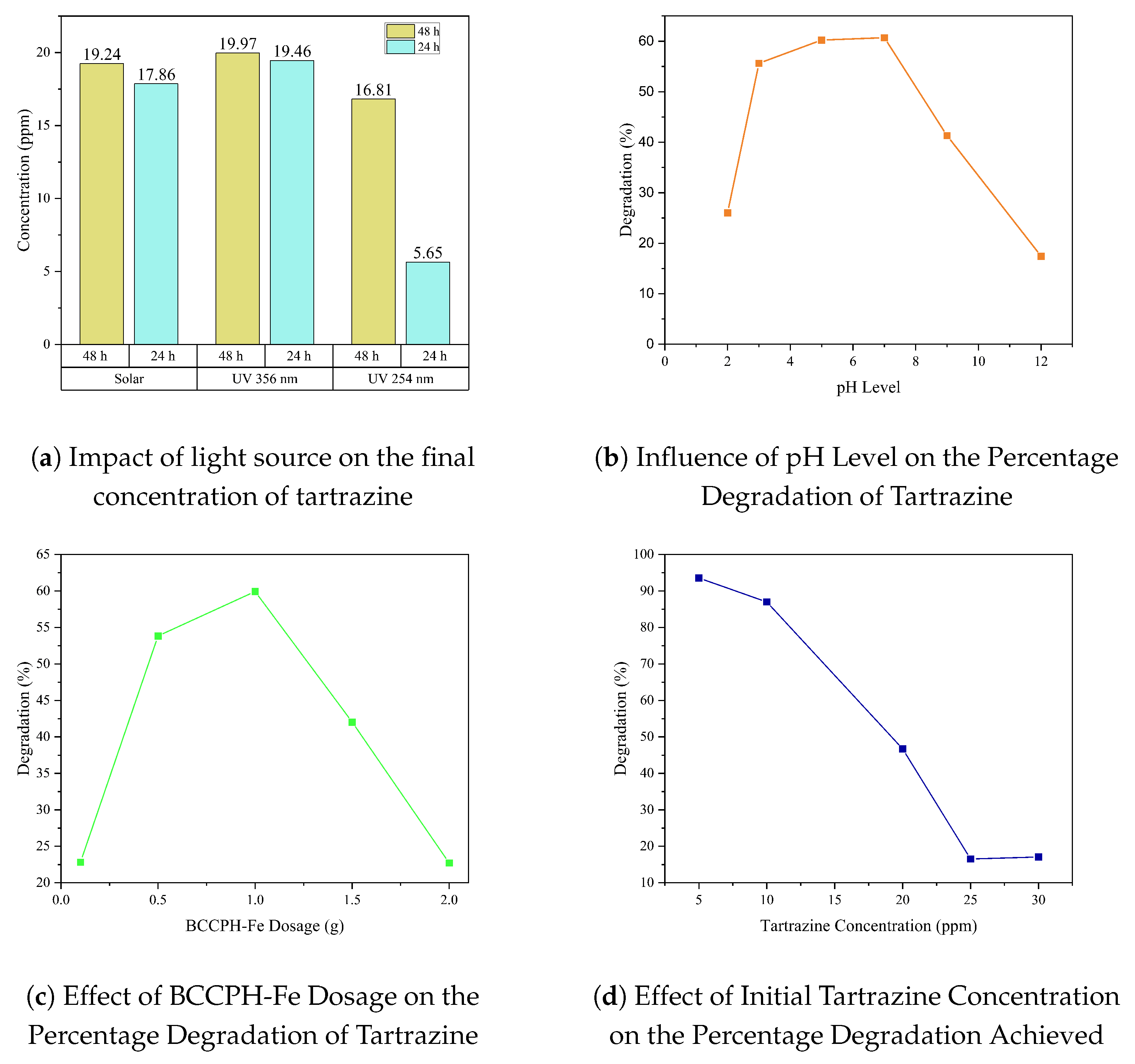
3.3.1. Equilibrium Studies
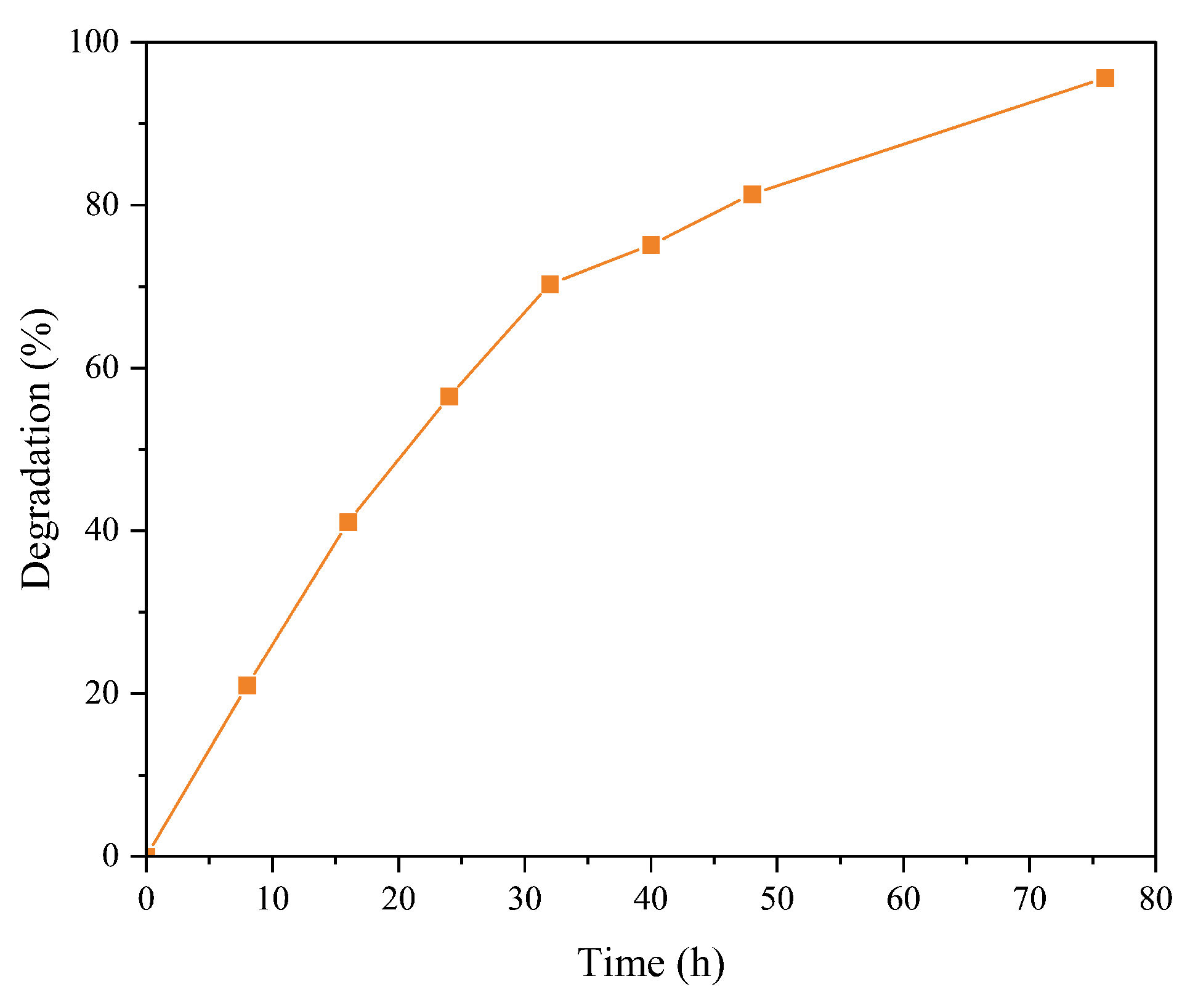
3.3.2. Kinetic Study
3.3.3. Study with the Best Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPH | Cocoa Pod Husk |
| CPHW | Cocoa Pod Husk Washed |
| BCCPH | BioChar from Cocoa Pod Husk |
| BCCPH-Fe | BioChar from Cocoa Pod Husk doped with Iron |
| MAP | Microwave Assisted Pyrolysis |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| BET | Brunauer-Emmett-Teller |
| TGA | Thermogravimetric Analysis |
| UV | Ultraviolet |
References
- Maheshwari, K.; Agrawal, M.; Gupta, A.B. Dye Pollution in Water and Wastewater. In Sustainable Textiles; Springer: Singapore, 2021; pp. 1–25. [Google Scholar]
- Ollis, D.F. Kinetics of Photocatalyzed Reactions: Five Lessons Learned. Front. Chem. 2018, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Spears, S. What Is Biochar? Regeneration International: Minneapolis, MN, USA, 2018. [Google Scholar]
- Reis, G.S.D.; Bergna, D.; Grimm, A.; Lima, É.C.; Hu, T.; Naushad, M.; Lassi, U. Preparation of Highly Porous Nitrogen-Doped Biochar Derived from Birch Tree Wastes with Superior Dye Removal Performance. Colloids Surfaces Physicochem. Eng. Asp. 2023, 669, 131493. [Google Scholar] [CrossRef]
- Correa-Abril, J.; Stahl, U.; Cabrera, E.; Parra, Y.; Vega, M.; Taamalli, S.; Louis, F.; Rodríguez-Díaz, J.M. Adsorption Dynamics of Cd2+ (Aq) on Microwave-Synthetized Pristine Biochar From Cocoa Pod Husk: Green, Experimental, and DFT Approaches. iScience 2024, 27, 109958. [Google Scholar] [CrossRef]
- Del Valle Morales, G.; Sham, E.L.; Cornejo, R.; Torres, E.M.F. Kinetic Studies of the Photocatalytic Degradation of Tartrazine. Lat. Am. Appl. Res. 2012, 42, 45–49. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, J.; Cabrera, E.V.; Stahl, U.; Correa-Abril, J. Kinetic and Equilibrium Analysis of Tartrazine Photocatalytic Degradation Using Iron-Doped Biochar from Theobroma cacao L. Husk via Microwave-Assisted Pyrolysis. Chem. Proc. 2024, 16, 52. https://doi.org/10.3390/ecsoc-28-20265
Espinoza J, Cabrera EV, Stahl U, Correa-Abril J. Kinetic and Equilibrium Analysis of Tartrazine Photocatalytic Degradation Using Iron-Doped Biochar from Theobroma cacao L. Husk via Microwave-Assisted Pyrolysis. Chemistry Proceedings. 2024; 16(1):52. https://doi.org/10.3390/ecsoc-28-20265
Chicago/Turabian StyleEspinoza, Jean, Elvia V. Cabrera, Ullrich Stahl, and Jhonny Correa-Abril. 2024. "Kinetic and Equilibrium Analysis of Tartrazine Photocatalytic Degradation Using Iron-Doped Biochar from Theobroma cacao L. Husk via Microwave-Assisted Pyrolysis" Chemistry Proceedings 16, no. 1: 52. https://doi.org/10.3390/ecsoc-28-20265
APA StyleEspinoza, J., Cabrera, E. V., Stahl, U., & Correa-Abril, J. (2024). Kinetic and Equilibrium Analysis of Tartrazine Photocatalytic Degradation Using Iron-Doped Biochar from Theobroma cacao L. Husk via Microwave-Assisted Pyrolysis. Chemistry Proceedings, 16(1), 52. https://doi.org/10.3390/ecsoc-28-20265






